Cytotoxicity Assessment Service
Comprehensive Cytotoxicity Assessment for Your Drug Development.
Cytotoxicity is a critical factor in drug development, determining whether a compound can cause harmful effects on healthy cells or tissues. For any therapeutic candidate, understanding the cytotoxic potential is essential to prevent adverse effects during clinical trials and ensure patient safety. Cytotoxicity assessment service at Creative Biolabs provides reliable, in-depth testing to help you evaluate the safety profile of your compounds with precision, ensuring that only the most promising candidates progress to the next phase of development.
Technical Methods for Cytotoxicity Assessment Service
|
Assay Type
|
Method
|
Purpose
|
|
Cell Viability Assays
|
MTT/MTS Assay
|
Measures cell metabolic activity as an indicator of cell viability.
|
|
XTT Assay
|
Similar to MTT, it uses a different reagent for better sensitivity.
|
|
Resazurin/Alamar Blue Assay
|
A fluorometric method for assessing live cell viability.
|
|
Apoptosis and Necrosis Detection
|
Annexin V/PI Staining
|
Differentiates apoptotic and necrotic cells.
|
|
Caspase Activity Assays
|
Detects caspase activation, a key marker for apoptosis.
|
|
Cell Proliferation Assays
|
BrdU Incorporation Assay
|
Measures DNA synthesis in proliferating cells.
|
|
Ki-67 Staining
|
Evaluates cell proliferation via a specific cell cycle marker.
|
|
Cell Morphology and Cytoskeletal Integrity
|
Fluorescent Microscopy
|
Detects changes in cell shape, attachment, and cytoskeletal organization.
|
|
Live/Dead Staining
|
Identifies live, dead, and membrane-compromised cells using fluorescent markers.
|
|
Genotoxicity Indicators
|
Comet Assay
|
Detects DNA strand breaks in individual cells.
|
|
γ-H2AX Foci Formation
|
Visualizes DDR activation by measuring γ-H2AX foci formation.
|
|
High-Content Screening (HCS)
|
Automated Imaging and Analysis
|
Assesses multiple cytotoxic endpoints (viability, morphology, apoptosis) in a single experiment.
|
Advantages of Our Cytotoxicity Assessment Service
-
Comprehensive and multi-dimensional testing
Our service combines various assay types, from simple cell viability to complex apoptosis, necrosis, and genotoxicity assessments. This provides a complete picture of your compound's cytotoxic effects.
-
Advanced technology integration
Utilizing HCS, live-cell imaging, and automated analysis, our assays provide highly sensitive, reproducible, and real-time data. This allows for a deeper understanding of how compounds interact with cells at multiple levels.
-
Early identification of safety issues
Our assays help identify potential cytotoxicity concerns early in the drug development process, allowing you to mitigate risks and prioritize safe candidates for further development.
-
In-depth mechanistic insights
With assays that explore apoptosis, necrosis, cell morphology, and DNA damage, our service not only measures cytotoxicity but also provides insights into the underlying mechanisms of cell death.
-
Customizable protocols
We tailor our cytotoxicity assays based on the specific needs of your compound type (small molecules, biologics, nanomaterials) and intended application, ensuring highly relevant results.
Applications
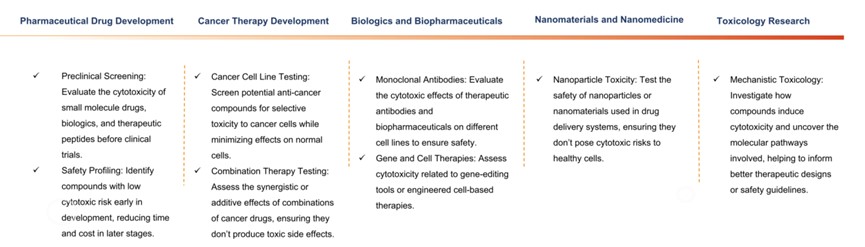
Frequently Asked Questions
Q1: What types of cells can be used in cytotoxicity testing?
A1: We offer testing on a wide range of cell types, including:
-
Cancer cell lines
-
Primary human or animal cells
-
Stem cells and induced pluripotent stem cells
-
Specialized cell lines for organ-specific toxicity
-
Custom cell lines can also be used upon request.
Q2: How much sample is required for cytotoxicity testing?
A2: The required amount depends on the specific assay type and scale. Typically, a few milligrams of a compound or small volumes of a solution are sufficient. For high-throughput studies, larger quantities may be necessary.
Q3: Can you assess both short-term and long-term cytotoxicity?
A3: Yes, we can perform both acute (24-48 hours) and chronic (up to several weeks) cytotoxicity studies depending on your requirements. Chronic studies are particularly relevant for assessing cumulative toxicity over time.
Q4: Can this service assess selective cytotoxicity?
A4: Yes, we can test selective cytotoxicity by comparing the effects of your compound on cancerous versus healthy cells or different cell types. This is especially useful for evaluating anti-cancer drugs or targeted therapies.
Q5: Can cytotoxicity testing be customized to specific project needs?
A5: We can tailor testing protocols, select specific assays, or include additional endpoints based on your project's objectives, compound type, and regulatory requirements.
Q6: How do you ensure the reliability and reproducibility of results?
A6: We use validated protocols, replicate testing, and advanced data analysis techniques to ensure accurate and reproducible results. Each assay includes positive and negative controls for quality assurance.
Cytotoxicity testing is essential to ensure the safety of your compounds in early-stage development and throughout clinical trials. Our cytotoxicity assessment service offers a wide range of advanced assays and tailored solutions, providing you with the insights needed to move forward with confidence. if you're ready to ensure the safety and success of your drug candidates, reach out to discuss how we can assist with your cytotoxicity testing needs.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure