Functional Analysis of Neoantigen-Specific TCR
With our Professional competence in functional analysis of neoantigen-specific TCR, Creative Biolabs has developed a comprehensive platform for neoantigen-based cancer immunotherapy development.
Introduction of Neoantigen-specific TCR
In contrast with conventional TCRs, as shown in Fig.1, TCR-like CARs or TCR-CARs based on neoantigens have the advantages of both TCRs and CARs. TCR-like CARs and TCR-CARs with a scFv targeting neoantigens could specifically target the tumor site and could be rapidly activated to offer robust and long-term protection. Creative Biolabs has established a comprehensive platform for the functional analysis of neoantigen-specific TCR.
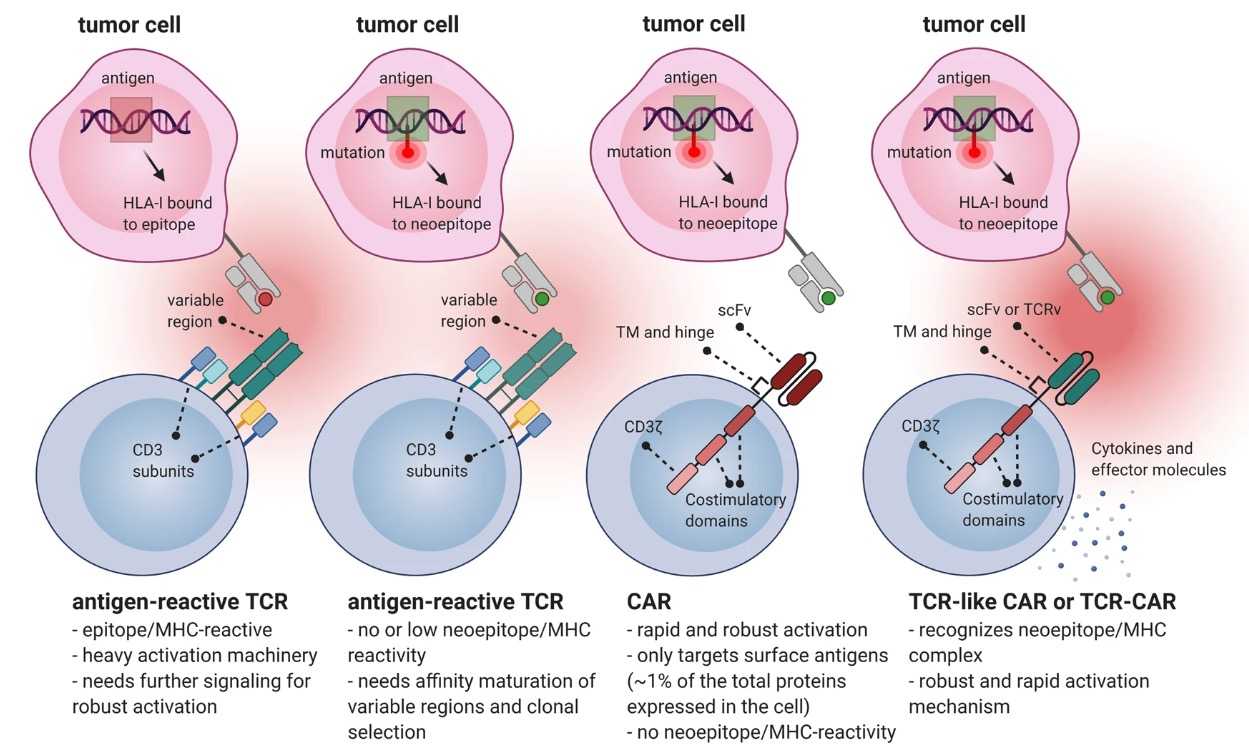 Fig.1 Advantages of Neoantigen-based TCR-like CAR or TCR-CAR.1
Fig.1 Advantages of Neoantigen-based TCR-like CAR or TCR-CAR.1
One-Stop Workflow for Functional Analysis of Neoantigen-specific TCR
Functional analysis of neoantigen-specific TCR is a routine program operated by a team of experts. With accumulated successful experiences, we could fulfill specific project requirements with quick responses. The typical workflow for our neoantigen-specific TCR analysis is shown in Fig.2.
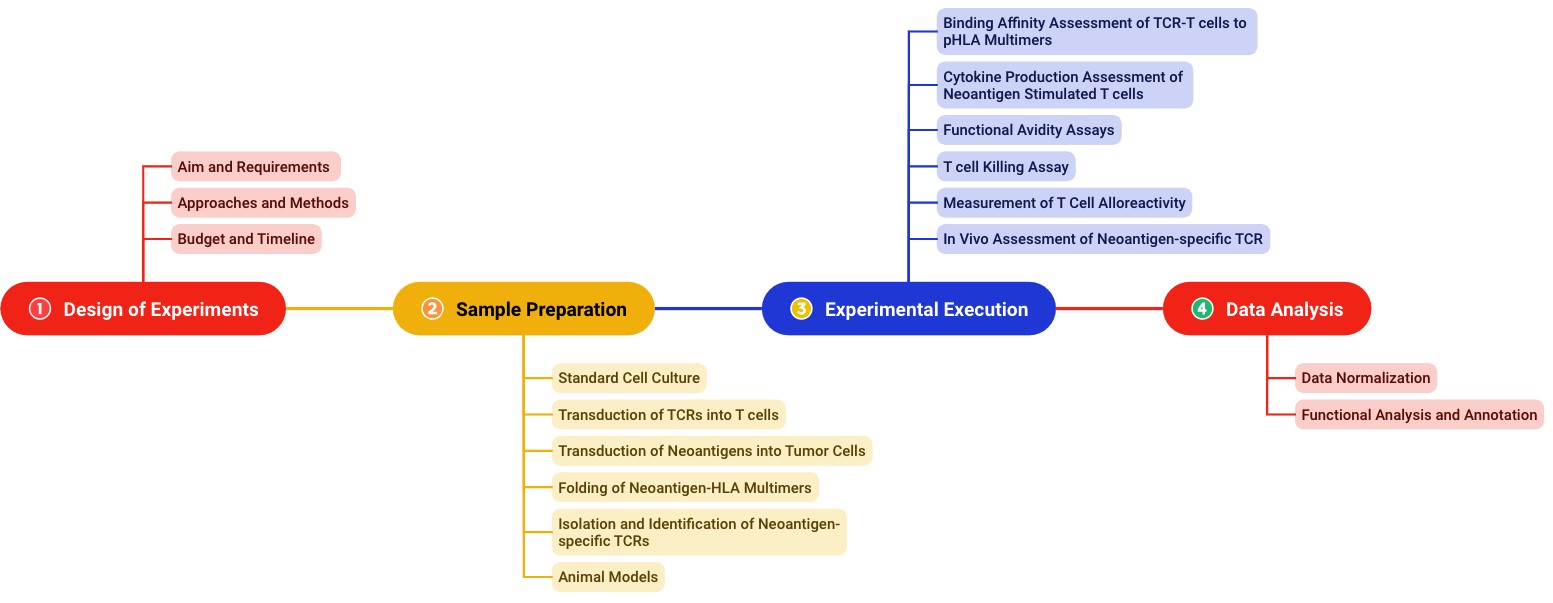 Fig.2 Experimental workflow for functional analysis of neoantigen-specific TCR.
Fig.2 Experimental workflow for functional analysis of neoantigen-specific TCR.
Key Features
-
Quick responses with a short timeline
-
High transduction efficiency to ensure cellular functional analysis
-
Comprehensive assessment routinely executed with a team of experts
-
Multi-dimensional analysis of the specificity, avidity, and longevity of neoantigen-specific TCRs
-
Professional data analysis with visual interpretation
Neoantigen-based immunotherapy has become a novel and ideal strategy for cancer treatment. For the advantages of neoantigen-specific TCR-like CARs and TCR-CARs, Creative Biolabs already has a professional and well-established platform to help our clients with the functional assessment of neoantigen-specific TCRs.
Reference
-
Poorebrahim, Mansour, et al. "TCR-like CARs and TCR-CARs targeting neoepitopes: An emerging potential." Cancer gene therapy 28.6 (2021): 581-589. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 Advantages of Neoantigen-based TCR-like CAR or TCR-CAR.1
Fig.1 Advantages of Neoantigen-based TCR-like CAR or TCR-CAR.1
 Fig.2 Experimental workflow for functional analysis of neoantigen-specific TCR.
Fig.2 Experimental workflow for functional analysis of neoantigen-specific TCR.
 Download our brochure
Download our brochure