NK Cell Culture Service for ATMP Development
Creative Biolabs provides a complete solution for generating large quantities of high-quality Natural Killer (NK) cells, essential for advancing your cell therapy projects. We deliver a scalable and reproducible process to ensure your project's success, from research to clinical-grade development. Our key problem-solving capabilities include overcoming the challenge of limited cell numbers in primary sources and mitigating the loss of cell viability and function during cryopreservation. We also provide comprehensive characterization data to meet regulatory requirements.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
The Role of NK Cells in Therapy
Natural Killer (NK) cells are a key component of the innate immune system and are a promising "off-the-shelf" option for cancer immunotherapy due to their ability to target and eliminate tumor cells without prior sensitization. The primary challenge in utilizing NK cells for adoptive cell therapies is obtaining a sufficient number of potent cells. Creative Biolabs' NK cell culture service leverages modern advancements in cell biology, including advanced feeder cell technology and optimized media, to address these challenges and provide a robust solution for the development of NK cell-based therapies.
For an in-depth analysis of how our services can be tailored to your specific project needs, request a consultation.
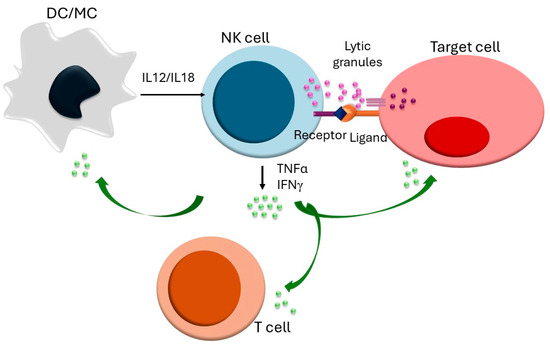 Fig.1 NK cell activation and immune regulation. 1
Fig.1 NK cell activation and immune regulation. 1
What We Can Offer
Efficient Expansion Systems
Our platform utilizes a proven feeder cell technology to facilitate a robust and significant expansion of NK cells, thereby ensuring you can secure a sufficient quantity of high-quality cells to meet the demands of your research needs.
Enhanced Functionality
Our service provides meticulously optimized cytokine cocktails, which are specifically formulated to enhance the anti-tumor functionality and prolong the in vitro persistence of NK cells, thereby supporting advanced research applications.
Superior Cell Recovery
We employ specialized cryopreservation protocols that are specifically developed to ensure superior post-thaw recovery and cell viability, which is essential for protecting the integrity of your cell product for future downstream experiments.
Custom Potency Assays
Our experts design and develop custom potency assays that are precisely tailored to meet your specific research objectives and provide the accurate, quantitative data required to successfully advance your program.
NK Cell Culture Service at Creative Biolabs
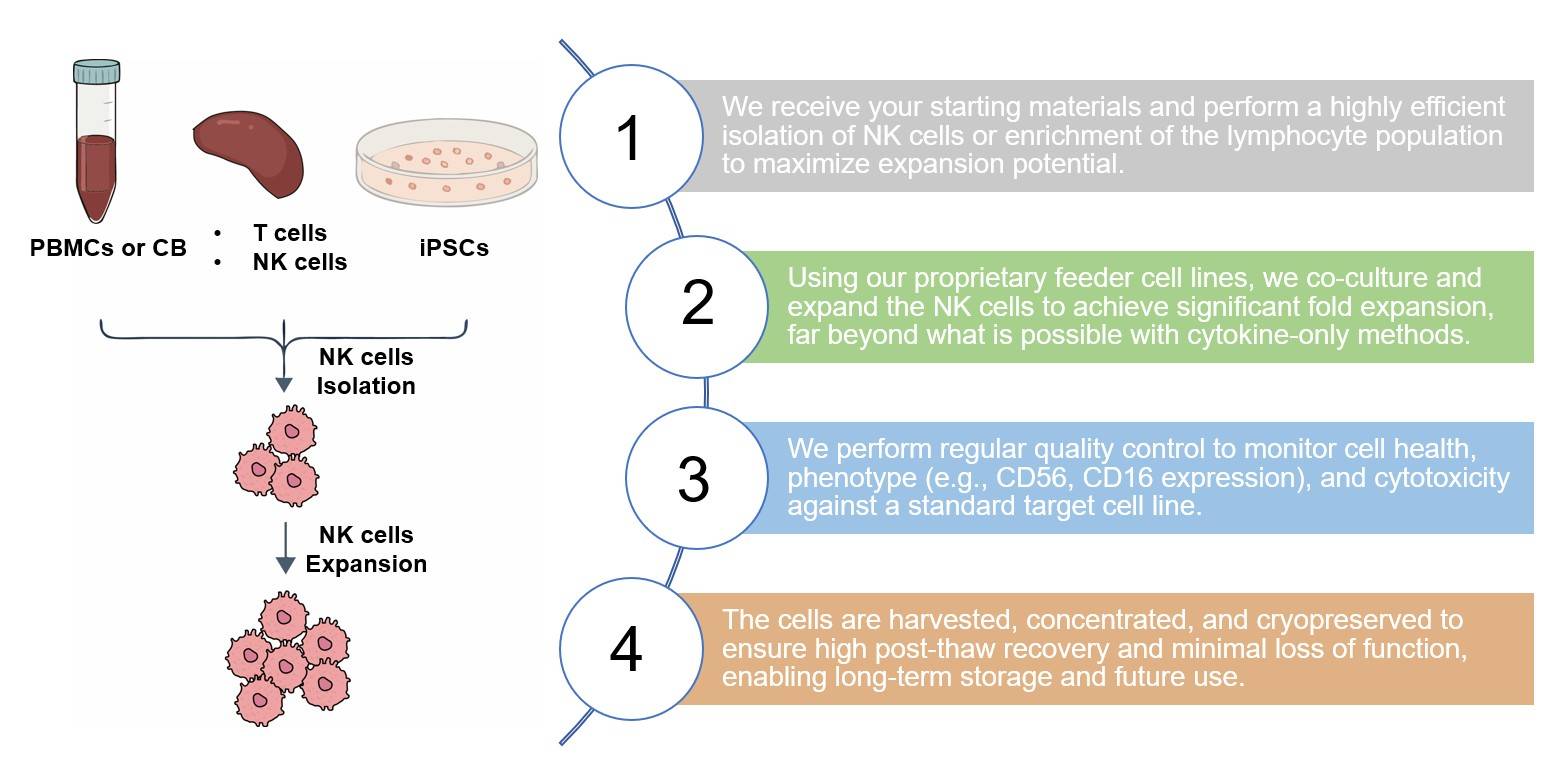
Highlights
Scientific Foundation
This service is predicated on a foundation of scientific rigor and advanced technological innovation. The methodologies and platforms employed are meticulously designed and validated to ensure high performance and to promote the success of therapeutic projects.
Proprietary Feeder System
A core distinguishing feature is the utilization of a proprietary feeder cell system. This technology is instrumental in achieving a substantial yield of expanded NK cells while simultaneously preserving their essential potency, which is a frequent challenge encountered in alternative expansion methodologies.
Preserving NK Cell Potency
The methodology places considerable emphasis on the preservation of NK cell potency, a critical functional attribute often compromised by less sophisticated protocols. This rigorous focus ensures that the cells maintain their high efficacy for their intended therapeutic applications.
Scalable & Compliant Processes
We specialize in providing a scalable, GMP-compliant process that is indispensable for the advancement of advanced therapy medicinal products (ATMPs). This expertise facilitates a streamlined transition from the research phase to clinical-grade production.
To discover the benefits of the Creative Biolabs platform by requesting a formal quotation, we invite you to get a quote today.
Customer Reviews
-
Functional Consistency
The potency assays provided with the service were instrumental. We confirmed that the expanded cells retained their superior cytotoxicity compared to our previous internal methods. This tangible data has been crucial for our research and publication efforts. - Jb C.
-
Simplified Expansion
We used to spend weeks on manual NK cell expansion, often with disappointing results. Creative Biolabs' service delivered a large, high-purity batch of functional NK cells that allowed us to move to in vivo studies much faster than we anticipated. - A***x D.
FAQs
Can your NK cell culture service be used for both autologous and allogeneic NK cell expansion?
Absolutely. Our flexible protocols are designed to accommodate a wide range of starting materials, including those for autologous (patient-derived) and allogeneic (donor-derived) cell therapy programs.
How do you ensure the expanded NK cells retain their anti-tumor functionality?
We go beyond simple cell counts. Our process includes comprehensive functional assays, such as cytotoxicity assays against relevant tumor cell lines, to confirm that the expanded cells are biologically active and potent. We believe this focus on function is critical for clinical success.
Related Services
iPSC Genetic Engineering
We offer advanced iPSC engineering services, utilizing technologies such as CRISPR/Cas9 to precisely modify genes, create knock-in or knock-out cell lines, and develop custom iPSC platforms for your specific research needs.
Learn More →
CAR-NK Engineering
Enhance the specificity and potency of your NK cells by engineering them to express chimeric antigen receptors (CARs) targeting specific tumor antigens.
Learn More →
How to Contact Us
At Creative Biolabs, we are dedicated to providing the highest quality NK cell products and services to help you bring your therapeutic vision to life. Our team of experts is ready to discuss your unique project requirements and demonstrate how our comprehensive service can accelerate your path to success. Please contact us.
Reference
-
Imširović, Vanna et al. "Maintaining the Balance: Regulation of NK Cell Activity." Cells vol. 13,17 1464. 31 Aug. 2024. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/cells13171464
For Research Use Only | Not For Clinical Use


 Fig.1 NK cell activation and immune regulation. 1
Fig.1 NK cell activation and immune regulation. 1


