Next-IO™ PD-1 x CTLA-4 Therapeutic Bispecific Antibody Program
About This Program
This program aims to develop PD-1 x CTLA-4 therapeutic bispecific antibody for melanoma immunotherapy.
Rationale
-
CTLA-4 and PD-1 are checkpoint receptors that highly expressed on tumor infiltrating lymphocytes (TILs).
-
TILs co-expressing multiple checkpoint receptors may be resistant to single checkpoint blockade.
-
Combination therapy in patients with advanced melanoma can significantly improve progression-free survival compared to each monotherapy alone.
-
Bispecific antibody (BsAb) is a novel antibody that mediates specific killing by targeting two different antigens and selectively redirecting effector cells to target cells. This enhanced synergistic anti-tumor effect highlights a promising approach to immunotherapy.
PD-1 x CTLA-4
PD-1 works as an immune-suppressing marker, and blocking PD-1/PD-L1 interaction plays a role in killing tumor cells, effective to inhibit tumor growth. Several anti-PD1 monoclonal antibodies have been approved by officials because they have great clinical anti-tumor outcomes.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, CD152), an immune checkpoint, is a membrane glycoprotein involving in the suppression of T cell immune functions like proliferation and cytokine production. CTLA-4 is a negative regulator of anti-tumor T cells upon recognition of their ligands. Currently, therapeutic antibodies targeting CTLA-4, ipilimumab, and tremelimumab, are approved to be efficient in a wide range of hematological malignancies and solid tumors.
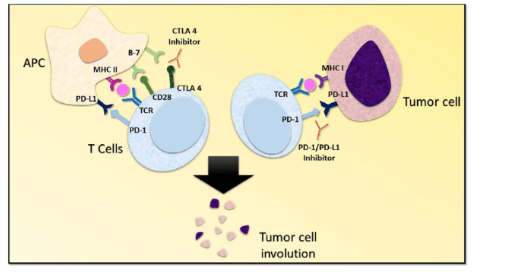 Fig.1 Mechanism of CTLA 4 and PD-1/PD-L1 inhibition.1
Fig.1 Mechanism of CTLA 4 and PD-1/PD-L1 inhibition.1
Published Data
The following data support the rationale for the development of PD-1 x CTLA-4 BsAbs with an improved therapeutic index for the treatment of tumor.
-
PD-1 x CTLA-4 bispecific antibodies promote in vivo T cell mediated anti-tumor efficacy.
Melanoma
-
Melanoma accounts for less than one percent of skin cancer cases but causes most skin cancer deaths. In the United States, the 5-year survival rate for patients with early melanoma findings is estimated to be approximately 98% in the United States. When the disease reaches the lymph node, the survival rate drops to 62%, and when the disease spreads to distant organs, the survival rate drops to 18%.
-
In the United States, about one person per hour dies of melanoma. It is estimated that 7,230 Americans will die of melanoma in 2019.
-
In 2019, more than 192,000 Americans are expected to be diagnosed with melanoma. Of these, more than 92,000 will be diagnosed as invasive.
Ongoing Clinical Trials
-
Up to now, only one anti-PD-1 x CTLA-4 BsAb is being studied in a clinical phase 1/2 trial for advanced solid tumors. Our program still holds broad market prospects.
|
NCT ID
|
Status
|
Sponsor
|
Project
|
Phase
|
Update Time
|
|
NCT03852251
|
Recruiting
|
Akeso
|
A Study of a PD-1/CTLA-4 Bispecific Antibody, for Advanced Solid Tumors or With mXELOX as First-line Therapy for Advanced Gastric or GEJ Adenocarcinoma
|
Phase 1/2
|
February 26, 2019
|
-
In an effort to optimally leverage PD-1 x CTLA-4-mediated immune response, our next-IO™ PD-1 x CTLA-4 targeted antibody program attempts to explore the optimal combination strategy by involving other immunomodulatory agents.
Program Management
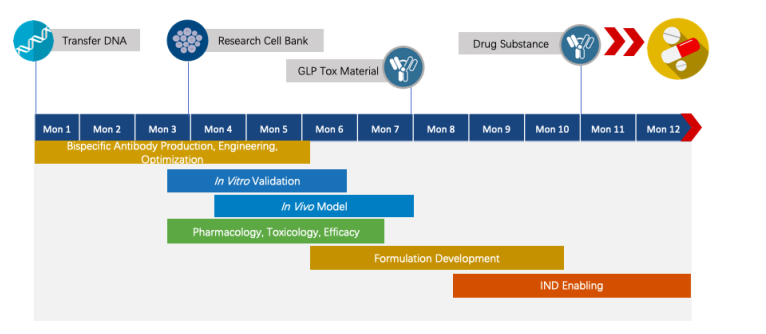
Creative Biolabs has extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop CD47 × GPC3 Dual-Targeted Fusion Protein program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
Reference
-
Chae, Young Kwang, et al. "Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC)." Journal for immunotherapy of cancer 6 (2018): 1-27. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 Mechanism of CTLA 4 and PD-1/PD-L1 inhibition.1
Fig.1 Mechanism of CTLA 4 and PD-1/PD-L1 inhibition.1

 Download our brochure
Download our brochure