Safety Panel Screening Service for Immuno-Oncology Drug Discovery
As the field of immuno-oncology continues to evolve, the need for comprehensive and reliable safety screening in drug discovery remains paramount. Creative Biolabs offers unparalleled expertise and a robust infrastructure to support researchers and pharmaceutical companies in the early identification of potential safety risks. We offer a comprehensive safety panel screening service, designed to streamline and de-risk the drug development process in the field of immuno-oncology.
Safety Panels
At Creative Biolabs, our safety panel screening service includes two distinct panels:
-
SafetyPanel44: This panel focuses on 44 clinically relevant targets, including G-protein-coupled receptors (GPCRs), ion channels, enzymes, etc. This panel aims for the early detection of potential safety issues by screening new compounds across these critical targets, thereby enhancing their safety profiles and reducing the risk of adverse reactions.
-
SafetyPanel90 Panel: SafetyPanel90 Panel: Building upon the foundation of SafetyPanel44, this panel includes 46 additional clinically relevant targets, providing comprehensive coverage of key physiological systems, including the central nervous system, cardiovascular system, metabolism, and immune response. By encompassing a broader spectrum of targets, it provides an in-depth analysis of drug safety by incorporating additional, clinically relevant targets, ensuring a more holistic safety evaluation.
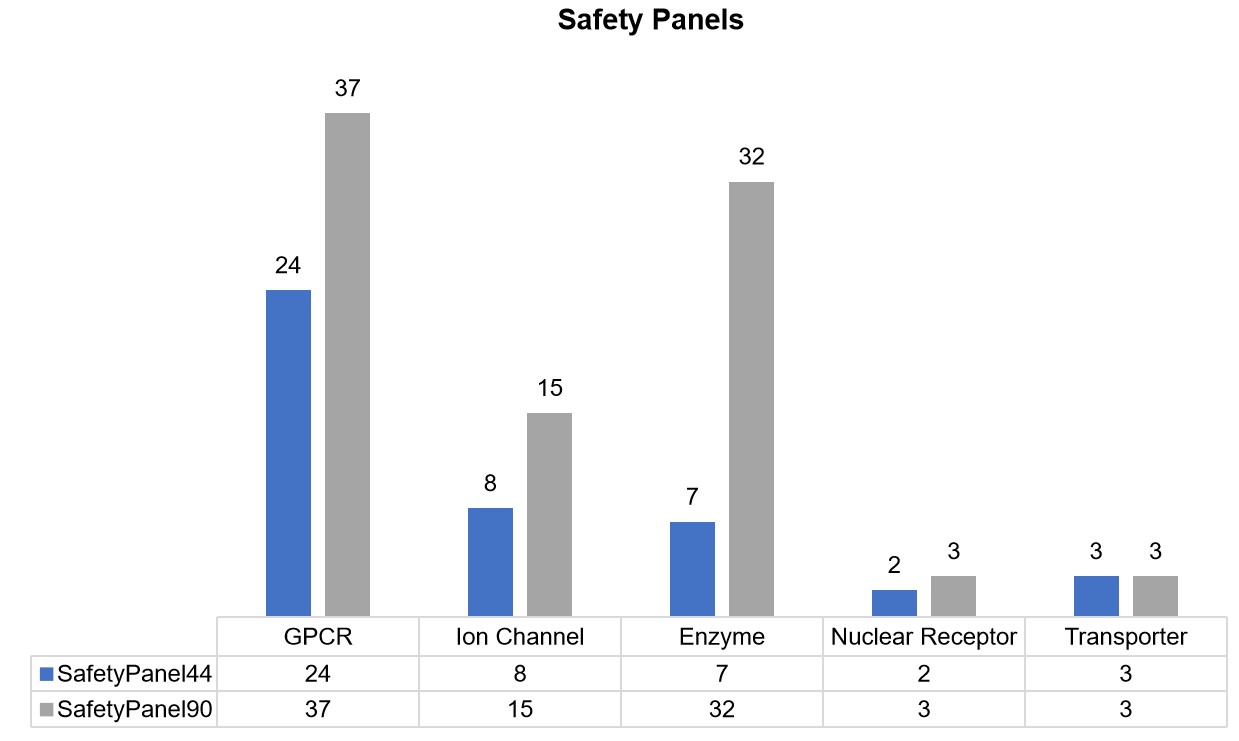 Fig.1 Safety panels and their targets.
Fig.1 Safety panels and their targets.
Advantages of Functional Assays: High Data Accuracy and Functional Relevance
At Creative Biolabs, we employ a variety of functional assays to provide results that correlate better with physiological and pharmacological functions, offering deeper insights into drug behavior.
-
Determine the final effects regardless of where the compound binds to the target.
-
Function-based screening focuses on the final functional outcome, resulting in significantly fewer false negatives and false positives compared to binding assays.
-
Distinguish between different modes of action (MOAs), such as agonistic and antagonistic effects.
-
Better suited for complex targets with multiple binding sites (e.g., ion channels).
-
Directly measure the EC50 of an agonist.
Service Highlights
-
Comprehensive Target Coverage: Screening against extensive panels, including both SafetyPanel44 and SafetyPanel90, ensures broad coverage of potential off-target interactions.
-
Identification of Agonists and Antagonists: Functional assays help differentiate between agonists and antagonists by evaluating their effects on target function, which is essential for understanding a compound's pharmacodynamics.
-
Rapid Turnaround Times: Creative Biolabs ensures prompt delivery of results within 4 weeks for the SafetyPanel44 and 6-8 weeks for the SafetyPanel90 Panel. This quick turnaround supports rapid decision-making during the drug discovery process.
Applications
-
Early Safety Risk Assessment
Our SafetyPanel44 and SafetyPanel90 enable early detection of off-target effects across vital systems such as the CNS, cardiovascular, and immune systems, helping de-risk drug candidates before clinical development.
-
Functional Pharmacology Profiling
Using function-based assays, researchers can evaluate compound activity (agonist or antagonist) on physiological outcomes, providing more accurate pharmacodynamic insights compared to traditional binding assays.
-
Accelerated Candidate Selection
By identifying compounds with favorable safety profiles early using SafetyPanel44 or SafetyPanel90, researchers can prioritize the most promising immuno-oncology candidates, reducing time and cost in the development pipeline.
-
Mode-of-Action Differentiation
Our functional assays distinguish between agonists and antagonists across complex targets like GPCRs and ion channels, offering critical insights into mechanism of action for safer and more targeted drug design.
Contact us today to discover how our safety panel screening services support your drug discovery endeavors.
For Research Use Only | Not For Clinical Use


 Fig.1 Safety panels and their targets.
Fig.1 Safety panels and their targets.
 Download our brochure
Download our brochure