T Cell Culture Service for ATMP Development
Creative Biolabs provides a specialized and comprehensive solution for addressing the complexities of T-cell culture in ATMP development. The service is meticulously designed to deliver a high-quality, scalable, and fully characterized T-cell product that not only fulfills a project's specific requirements but also adheres to the stringent regulations of governing bodies. Functioning as a seamless extension of a client's R&D team, this service provides the requisite expertise, technology, and compliance framework to facilitate the transition of therapeutics from laboratory research to clinical application.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
Why We Culture T Cells
T-cell culture and expansion are foundational to the development of immunotherapies. This complex process involves isolating, activating, and expanding T-cells to a therapeutic dose while preserving their anti-tumor functionality. Our service is built on decades of research showing that controlled and optimized ex vivo conditions are critical for generating a potent and durable cell product. This scientific foundation allows us to provide a service that mitigates the risks of T-cell exhaustion and ensures a product with the desired memory and effector phenotypes.
To explore how our services can be leveraged to address your specific project requirements, request a consultation.
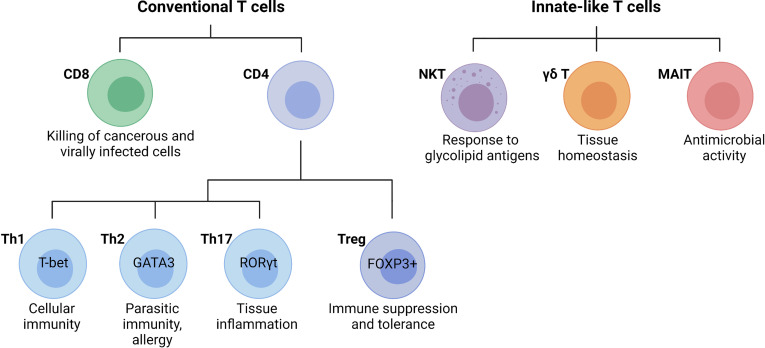 Fig.1 Main T cell subsets and their functions.1
Fig.1 Main T cell subsets and their functions.1
What We Can Offer
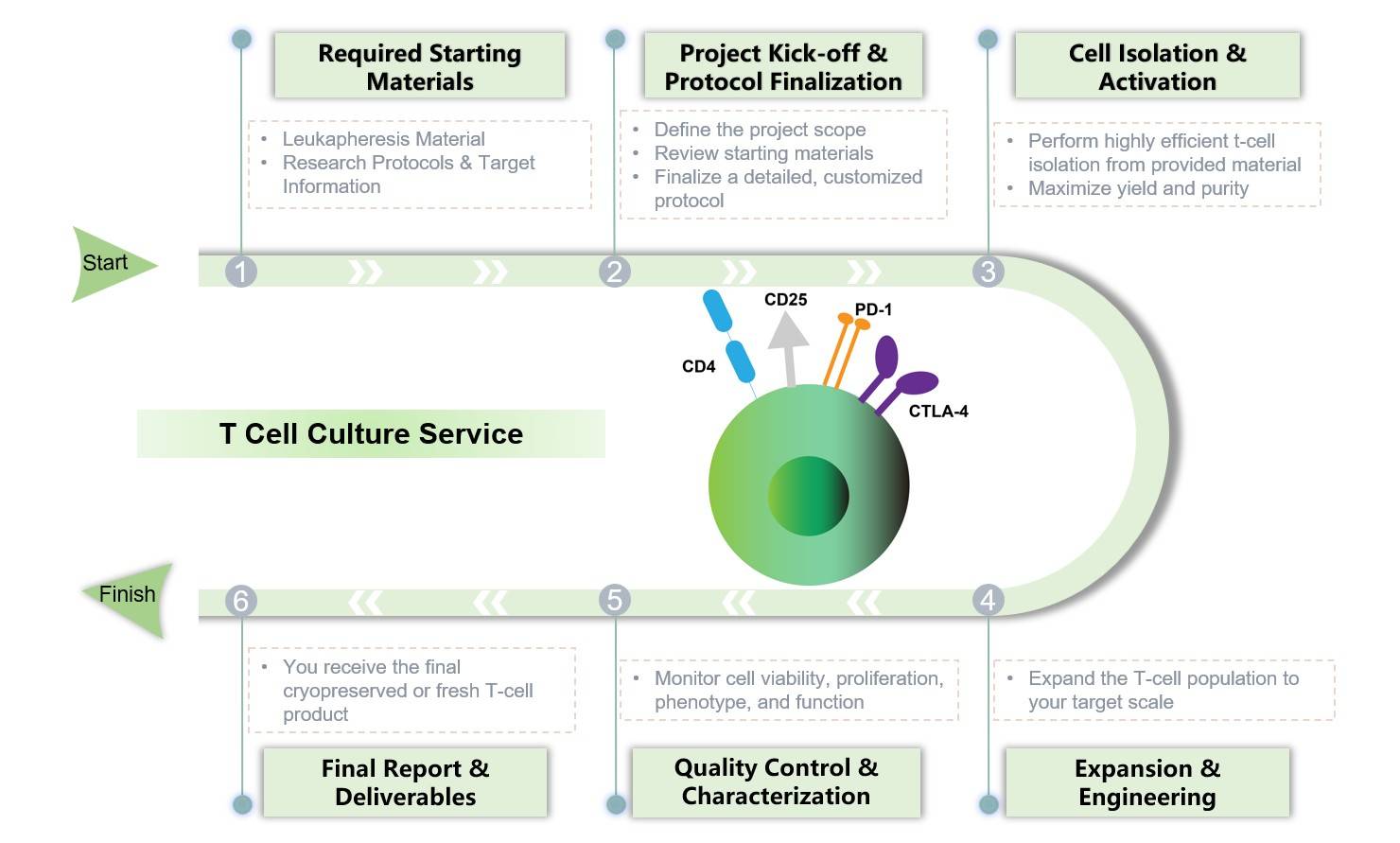
Our T-cell culture and manufacturing services are built upon a foundation of standardized, robust, and scalable processes for T-cell isolation, activation, and expansion. This platform ensures consistent, high-quality cell production for every project. The platform also incorporates a range of optimized viral and non-viral genetic modification methods, providing the flexibility to implement various engineering strategies to meet the specific requirements of your therapeutic program.
The manufacturing capabilities are designed for complete scalability. This ensures a seamless transition as your therapy advances through the development pipeline. Furthermore, each project includes a comprehensive and customized QC testing package, which is specifically designed for T-cell products to verify critical quality attributes such as purity, viability, and potency.
T Cell Culture Service at Creative Biolabs
Highlights
Scientific Expertise
The distinct advantage of partnering with Creative Biolabs is derived from the integration of extensive scientific experience with a platform-based methodology. This foundational approach culminates in the delivery of a product that is both potent and optimized for regulatory readiness.
Accelerated Timelines
Our platform-based methodology is instrumental in providing a notable advantage by substantially reducing the duration required to transition a therapeutic from research to clinical application. This streamlined process serves to ameliorate typical impediments and expedite the overall development lifecycle.
Tailored Solutions
Our collaborative approach enables the development of highly customized protocols to meet the unique requirements of each project. We offer flexibility in key parameters, from cell sourcing and genetic modification to final product specifications.
Optimized Product Potency
The synergistic application of our scientific experience and rigorous process development is essential for the consistent production of a high-quality therapeutic. This approach is instrumental in delivering a product that is consistently potent, functional.
To fully comprehend the distinct advantages offered by Creative Biolabs, we invite you to get a quote today.
Customer Reviews
-
Streamlined Process
The ability to offload T-cell expansion to Creative Biolabs has freed up our internal resources, allowing us to focus on downstream analytics and disease modeling. Their communication and transparency throughout the project were excellent. - Ca Ly**
-
High−Quality Product
The purity and functional potency of the T-cell product we received were outstanding. We were able to move our in vitro studies to a successful in vivo trial much faster than anticipated, thanks to the quality of the cells. - Dr. Sn Rs**
FAQs
What is the minimum number of cells required to start a project?
Our service is designed to be flexible. We can work with a wide range of starting cell numbers, but we typically recommend a minimum based on your final yield goals. Please contact our team to discuss your specific needs for an accurate assessment.
How does your service compare to in-house T-cell culture?
While in-house culture provides control, it comes with high capital costs, extensive training needs, and the risk of batch-to-batch variability. Our service provides a cost-effective, standardized, and validated alternative that accelerates your timeline and allows you to focus on your core therapeutic innovation.
Related Services
Cell Line Characterization
Comprehensive characterization and validation of your cell lines are critical for safety and regulatory compliance. Our services include identity verification, genetic stability testing, and mycoplasma detection to ensure the integrity and reliability of your cellular starting materials.
Learn More →
TCR/CAR Expression Analysis
Verify the success of your genetic engineering with our specialized TCR/CAR expression analysis services. We offer comprehensive validation of both gene and protein expression using advanced techniques such as flow cytometry, Western blot, and ELISA, ensuring the functionality and quality of your T-cell product.
Learn More →
How to Contact Us
Creative Biolabs provides standardized, scalable, and GMP-compliant platforms that free you from the complexities of cell culture, allowing you to focus on developing life-changing therapies. To discuss your project and discover how Creative Biolabs can support your goals, please contact us.
Reference
-
Hirsova, Petra et al. "Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma." Frontiers in endocrinology vol. 12 760860. 28 Oct. 2021. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fendo.2021.760860


 Fig.1 Main T cell subsets and their functions.1
Fig.1 Main T cell subsets and their functions.1


