Tumor-Infiltrating Lymphocyte (TIL) Culture Service for ATMP Development
Creative Biolabs provides a comprehensive, integrated solution for the delivery of a high-quality, patient-specific TIL product. This service encompasses a robust workflow that meticulously manages the entire process, thereby ensuring consistency and quality throughout every phase. The process involves the expert handling of the intricate and critical phases of TIL isolation and expansion, which enables client teams to concentrate on downstream therapeutic development and clinical strategy. The final deliverable is a potent, well-characterized product, meticulously prepared for direct integration into your advanced therapeutic medicinal product (ATMP) program.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
The Role of TILs in Immunotherapy
Tumor-infiltrating lymphocytes (TILs) are the body's naturally occurring immune cells that constantly patrol for and attack cancer cells. These cells uniquely recognize tumors through patient-specific markers on the cancer cell surface. This personalized recognition makes TILs a powerful tool, as they can combat the diverse tumor markers that enable cancer to evade the immune system. However, tumors can exhaust these TILs, rendering them ineffective. TIL therapy aims to overcome this challenge by expanding the patient's exhausted TILs into a robust army, re-equipping the immune system to fight back.
Initiate a consultation for a comprehensive discussion of project alignment.
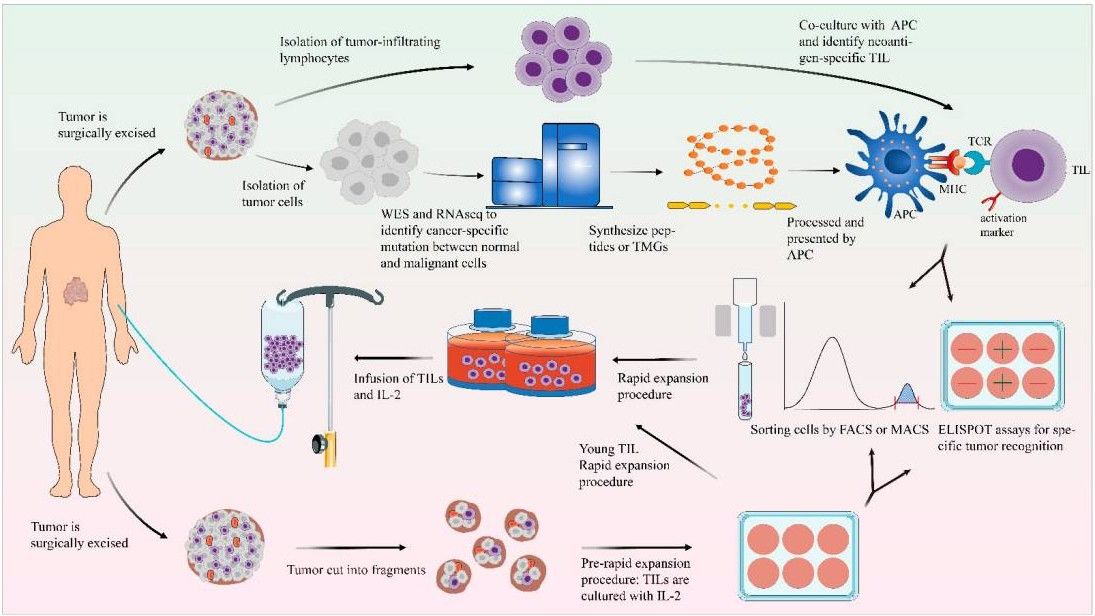 Fig.1 General scheme of the generation of TILs.1
Fig.1 General scheme of the generation of TILs.1
What We Can Offer
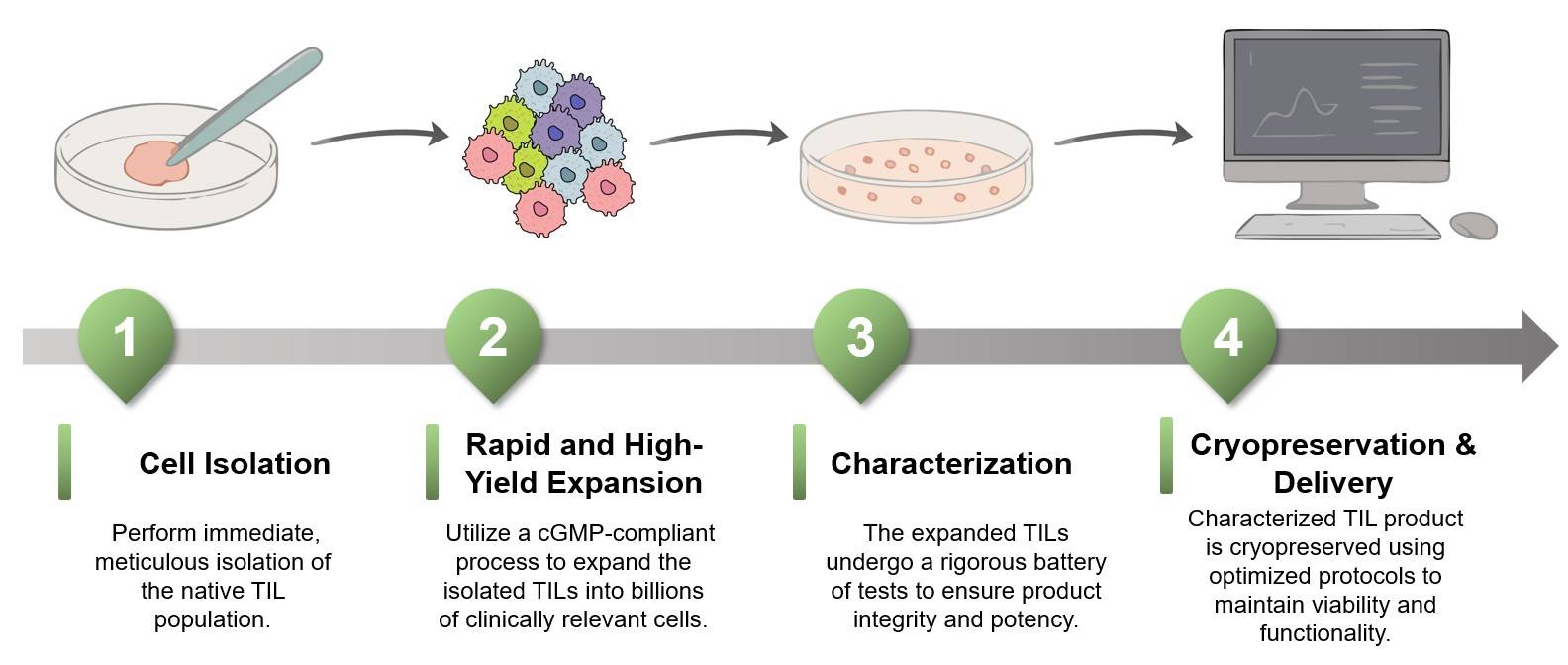
End-to-End Service
We provide a comprehensive, one-stop solution for TIL culture, managing every step from initial isolation to final cryopreserved product delivery. This ensures a seamless and efficient workflow for your project.
High-Yield Process
Our efficient upstream and downstream process development is meticulously designed to accelerate your ATMP pipeline. This focus on maximizing cell yield ensures we meet your project's aggressive timelines and production goals.
Customized Protocols
We create tailored protocols and culture conditions to maximize both TIL yield and potency. Our approach is uniquely optimized to address the specific needs and challenges of your individual project.
Rigorous Quality Control
Our well-established quality system and rigorous control tools ensure the stability and functionality of your final TIL product. We guarantee that every batch meets the highest standards for use in clinical applications.
Tumor-infiltrating Lymphocyte Culture Service at Creative Biolabs
Highlights
Strategic Partnership
Choosing Creative Biolabs provides a distinct strategic advantage for your ATMP program. Our service is purpose-built specifically for TILs, offering a specialized solution unlike generalized offerings. We are committed to turning scientific potential into a reliable therapeutic product.
Proven Track Record
We have a demonstrated track record of successfully culturing TILs from difficult-to-treat solid tumors. Our team has achieved an impressive 95% success rate in isolating and expanding TILs from pancreatic cancer specimens, validating our capabilities in challenging cases.
Specialized Expertise
Our deep expertise in cellular biology and TIL-specific protocols allows us to succeed where other labs may have failed. We understand the nuances of TIL isolation and expansion, ensuring a viable and potent cell product for your research and development efforts.
Competitive Edge
By partnering with Creative Biolabs, you gain a competitive edge in the complex field of cell therapy. Our specialized focus and dedication to TILs ensure that your project receives the highest level of care and precision from start to finish.
To fully understand the Creative Biolabs advantage, we invite you to get a quote today.
Customer Reviews
-
Logistical Advantage
The streamlined process for shipping and receiving tumor samples was a huge advantage. They took care of the complex logistics and provided a clear, step-by-step workflow, which was a major relief for our team. Their service facilitated a much faster research timeline. - Ly Rd.
-
Functional Validation
We were particularly impressed with the comprehensive characterization data provided by Creative Biolabs. The functional assays confirmed the anti-tumor activity of the expanded TILs, giving us the confidence we needed to move into our next development phase. - Am Bs.
FAQs
What is the optimal type of tumor sample to provide?
We prefer fresh, surgically resected tumor tissue, as this typically yields the highest number of viable TILs. If you have a different type of sample, please contact us to discuss the specifics. We're happy to assess your project's feasibility.
How do you ensure the expanded TILs are functional?
We perform comprehensive functional assays, including cytotoxicity assays against relevant tumor cell lines and cytokine profiling. This ensures the final product is not only numerous but also potent and capable of killing cancer cells.
Related Services
Contamination Testing
This critical quality control service ensures the final cell product is free from mycoplasma contamination, which is essential for the safety and integrity of patient-centric ATMP development.
Learn More →
Cytokine Measurement
Creative Biolabs provides detailed profiles of cytokine and chemokine secretion from your expanded TIL population, offering a deeper understanding of their functional state and potential anti-tumor activity.
Learn More →
How to Contact Us
Creative Biolabs provides the expertise, the technology, and the uncompromising quality control needed to ensure your TIL-based therapy realizes its full potential. To discuss how our TIL culture service can accelerate your ATMP development pipeline, please contact us.
Reference
-
Zhao, Yueshui et al. "Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges." Cancers vol. 14,17 4160. 27 Aug. 2022. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/cancers14174160
For Research Use Only | Not For Clinical Use


 Fig.1 General scheme of the generation of TILs.1
Fig.1 General scheme of the generation of TILs.1


