Tumor Model Construction Service for Cancer Metabolism Research
Background Service Highlights FAQs Contact Us
Introduction: The Metabolic Revolution in Oncology
Cancer is fundamentally a disease of aberrant cellular energetics. For decades, the focus of oncology has been on the genetic mutations that drive uncontrolled cell proliferation. However, a paradigm shift is underway, recognizing that to fuel this relentless growth, cancer cells must profoundly rewire their metabolic pathways. This metabolic reprogramming is no longer seen as a mere consequence of malignancy but as a central hallmark, offering a rich landscape of novel therapeutic targets. Cancer cells exhibit remarkable metabolic plasticity, altering pathways such as glycolysis, oxidative phosphorylation (OXPHOS), and the metabolism of glutamine and fatty acids to meet the bioenergetic and biosynthetic demands of tumor progression, invasion, and resistance to therapy.
To effectively exploit these metabolic vulnerabilities, researchers require experimental systems that transcend traditional, simplistic models. It is imperative to study cancer metabolism within a context that faithfully recapitulates the physiological complexity of the tumor microenvironment (TME), with its inherent nutrient gradients, hypoxia, and intricate cell-cell interactions. At Creative Biolabs, we leverage over two decades of specialized expertise to construct sophisticated, physiologically relevant tumor models that serve as powerful tools for dissecting the metabolic intricacies of cancer and accelerating the development of next-generation oncology therapeutics.
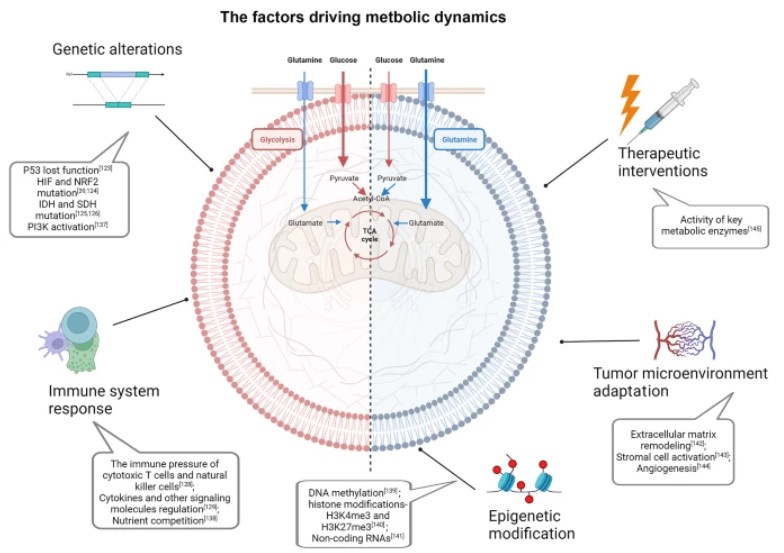 Fig.1 The factors driving metastatic dynamics of cancer cells.1
Fig.1 The factors driving metastatic dynamics of cancer cells.1
Our Tumor Model Construction Service for Cancer Metabolism Research
Understanding the metabolic dependencies of a tumor is critical for identifying effective therapeutic strategies. However, the metabolic profile of cancer cells cultured on a flat, plastic dish often bears little resemblance to their behavior within a living organism. Standard models fail to replicate the selective pressures of the TME that force cancer cells to adapt their metabolism for survival. Our specialized service focuses on building bespoke in vitro and in vivo models designed specifically for the nuanced demands of cancer metabolism research. These models provide a robust platform to investigate how tumors acquire and utilize nutrients, identify key metabolic fluxes that are essential for their growth, and test the efficacy of compounds designed to disrupt these critical pathways.
By using models that accurately reflect in vivo conditions, researchers can gain high-confidence insights into target engagement and therapeutic efficacy. For instance, investigating the Warburg effect—the reliance of many tumors on aerobic glycolysis—requires a model with a realistic glucose gradient. Similarly, studying the role of glutaminolysis or fatty acid oxidation in metastasis demands a system that incorporates the complex interactions between tumor cells and stromal components. Creative Biolabs provides these essential, context-rich environments.
A Tailored Portfolio of Advanced Tumor Models
We offer a comprehensive suite of tumor model construction services, utilizing cutting-edge techniques to create models that are customized to the specific needs of each research project.
Advanced In Vitro Models
-
3D Spheroid and Organoid Cultures: Moving beyond the limitations of 2D monolayers, we construct three-dimensional spheroids and patient-derived organoids. These models mimic the 3D architecture of solid tumors, establishing natural gradients of oxygen and nutrients. This is critical for studying metabolic zonation within a tumor, where cells in a hypoxic core may rely on different metabolic pathways than those at the well-oxygenated periphery.
-
Tumor-Microenvironment-on-a-Chip (TMOC): Employing state-of-the-art microfluidics, we build microphysiological systems that simulate the TME with unparalleled fidelity. These "on-a-chip" models can incorporate perfusable vascular channels, stromal cells (e.g., fibroblasts), and even circulating immune cells, allowing for the dynamic study of metabolic crosstalk between different cell populations.
-
3D Bioprinting: For maximum precision and architectural control, Creative Biolabs utilizes 3D bioprinting to deposit living cells and biomaterials with precise spatial arrangements. This technique enables the creation of highly structured and reproducible tumor models that can replicate complex tissue interfaces, ideal for studying invasion and the metabolic changes that accompany it.
High-Fidelity In Vivo Models
-
Patient-Derived Xenograft (PDX) Models: As leaders in translational oncology models, we establish PDX models by directly implanting patient tumor tissue into immunocompromised mice. These models are the gold standard for personalized medicine research, as they preserve the genetic heterogeneity, histopathology, and most importantly the specific metabolic signature of the original human tumor. They provide the most clinically relevant platform for testing metabolic inhibitors.
-
Syngeneic and Genetically Engineered Mouse Models: For studies requiring a fully competent immune system, we utilize syngeneic models (transplanted tumors in immunocompetent mice) and genetically engineered mouse models. These models are indispensable for investigating the interplay between cancer metabolism and the host immune response, a critical area for the development of combination therapies involving immunotherapies and metabolic inhibitors.
Our Advantages
Choosing Creative Biolabs provides a distinct advantage born from deep scientific expertise and a commitment to collaborative partnership.
-
Unmatched Expertise
-
Precision and Customization
-
Integrated Analytical Services
FAQs
Q1: Which tumor model is most appropriate for my drug discovery project?
A1: The optimal model depends on your specific research question and therapeutic agent. For early-stage screening, 3D spheroids may be ideal. For validating a lead compound targeting a specific metabolic pathway in a patient-relevant context, a PDX model is often the best choice. Our experts will work with you to determine the most suitable model system.
Q2: How do you ensure the reproducibility and relevance of your models?
A2: We employ rigorous quality control measures at every stage of model construction. For PDX models, we perform comprehensive genetic and phenotypic characterization to ensure fidelity to the original patient tumor. For our in vitro models, we use standardized protocols and advanced techniques like 3D bioprinting to ensure structural and cellular reproducibility.
Q3: Can your models be used to study the metabolic interplay between cancer cells and immune cells?
A3: Absolutely. Our syngeneic models and advanced TMOC systems, which can incorporate immune cells, are specifically designed for this purpose. These models are powerful tools for understanding how tumor metabolism influences the immune microenvironment and for testing the efficacy of novel immunometabolism-targeted therapies.
Contact Us
Advance your cancer metabolism research with models that provide true physiological relevance. Connect with the scientific team at Creative Biolabs today to discuss your project and discover how our expertise in tumor model construction can help you achieve your research and development goals. Let us be your partner in translating metabolic insights into life-saving therapies.
Reference
-
Yang, Jianqiang et al. "Cancer metabolism and carcinogenesis." Experimental hematology & oncology vol. 13,1 10. 29 Jan. 2024. DOI: 10.1186/s40164-024-00482-x. Distributed under Open Access License CC BY 4.0, without modification.


 Fig.1 The factors driving metastatic dynamics of cancer cells.1
Fig.1 The factors driving metastatic dynamics of cancer cells.1
