Post-Translational Modification (PTM) Analysis Service
Therapeutic monoclonal antibodies (mAbs) may undergo post-translational modifications (PTMs), resulting in significant heterogeneity in the modified mAbs. Creative Biolabs has established a comprehensive mass spectrometry (MS) monitoring platform to provide our customers with rapid, accurate, and sensitive PTM analysis.
PTMs and mAbs
PTMs increase the complexity of the protein repertoire and lead to significant heterogeneity in mAbs subjected to different treatments, and some modifications may pose considerable challenges to the biological activity, stability, and safety of therapeutic mAbs. Through high-resolution mass spectrometry platforms, we offer reliable detection and unbiased identification of these emerging modified biomarkers.
Sample Submission
We sincerely encourage you to deliver samples in the form of 2D gel spots, PAGE bands, or purified proteins for detection purposes. We also possess the capability to handle protein samples obtained in any desired form like cultured cells or biological tissues. To ensure the utmost precision in PTM detection, information regarding sample processing procedures, buffer compositions, and any relevant details about the protein/antibody would be appreciated.
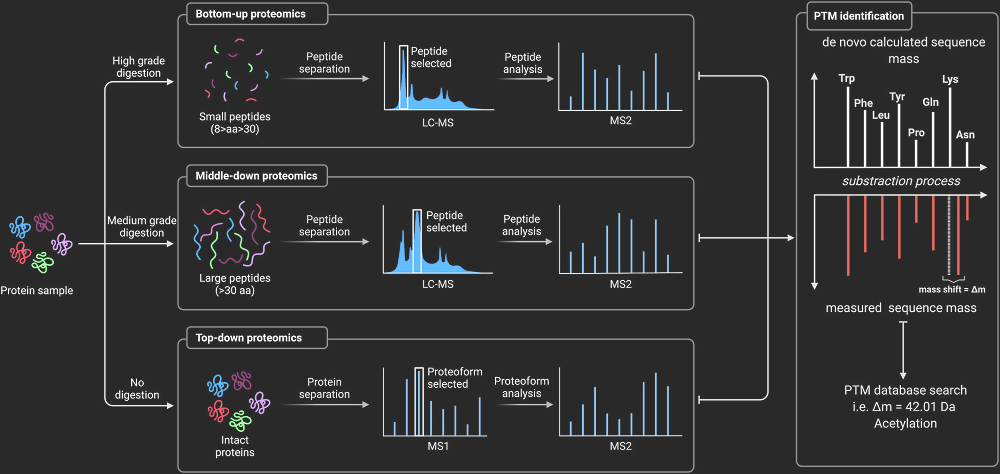 Fig 1. MS/MS PTM analysis.
Fig 1. MS/MS PTM analysis.
PTM Analysis Approaches
We recommend using LC-MS/MS for sensitive, accurate, and efficient PTM analysis. Our team of experts will meticulously evaluate your project requirements, sample complexity, protein sequence coverage, desired sensitivity, and detection limits to select the appropriate PTM detection strategy (bottom-up, top-down, middle-down proteomics).
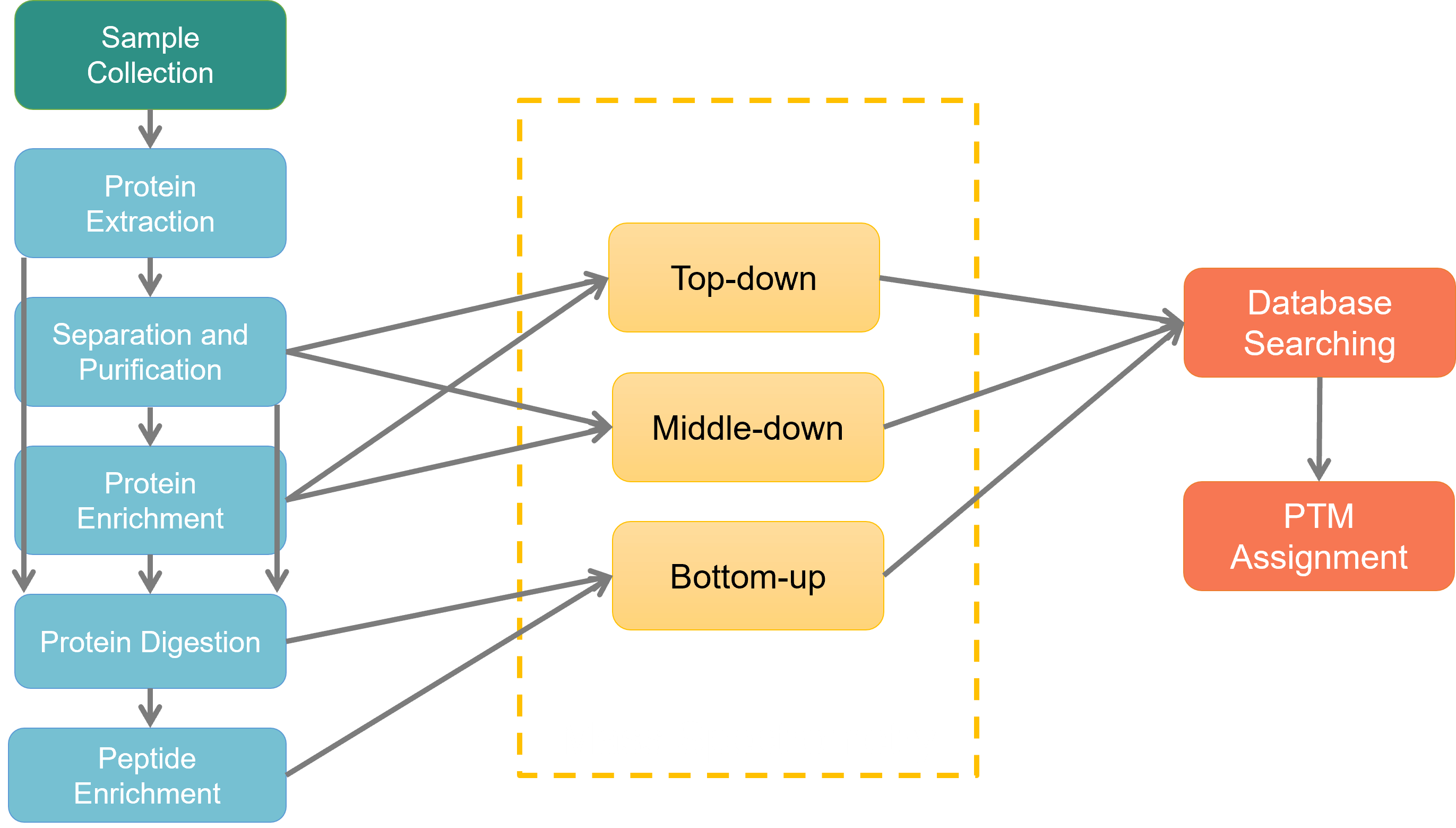 Fig 2. Workflow of PTM analysis.
Fig 2. Workflow of PTM analysis.
What We Provide
- Creative Biolabs is delighted to offer comprehensive PTM analysis services, encompassing the recognition of commonly occurring PTM modifications, antibody modification determination, broad-spectrum analysis of PTM-modified proteins as well as the identification of specific site/target protein modifications.
- The MS/MS spectra will be searched and compared against protein databases to identify all potential combinations if there is no specific additional requirement. We are committed to delivering prompt data turnover and thorough analysis processing.
- Your samples will be diligently analyzed within three weeks with a complete data report.
- We possess the expertise to undertake PTM modification de novo sequencing for more complex or unknown sequences.
Our Services
PTM analysis involves a diverse range of technologies, processes, and outcome deliverables, catering to distinct testing needs. Creative Biolabs invites you to initiate connect with our experienced technical team for a detailed discussion of your specific research requirements. Our experts are dedicated to providing optimal solutions precisely tailored to your needs.
Reference
- Hermann, J., Schurgers, L., & Jankowski, V. (2022). Identification and characterization of post-translational modifications: Clinical implications. Molecular Aspects of Medicine, 86, 101066.