Based on the CellRapeutics™ platform, Creative Biolabs offers a range of CAR-T effectiveness testing services. Hopefully, the following information will be helpful for your research project.
The challenges of CAR-T cell therapy are mainly related to side effects, toxicity, T cell depletion, and malignant tumor microenvironment (TME). With the emergence of more and more CAR-T products, the importance of quality control and effectiveness has gradually become prominent. Among them, the effectiveness test is mainly used to judge the efficacy of CAR-T products. Studies have shown that the efficacy of CAR-T cells is related to a variety of factors, including CAR structure, transfection efficiency, gene vector, cell type, and cell ratio.
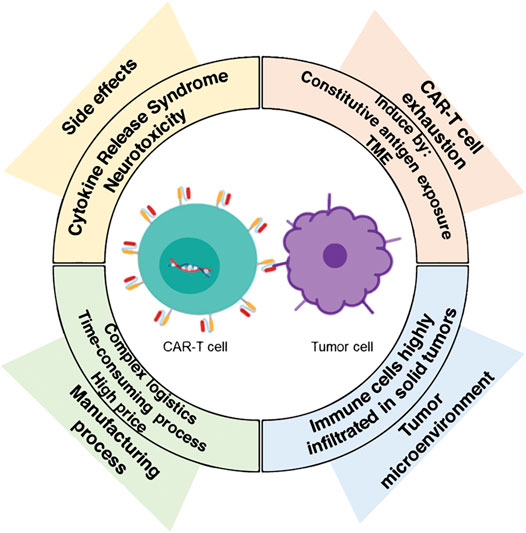 Fig.1 Challenges for CAR-T cell therapy.1,3
Fig.1 Challenges for CAR-T cell therapy.1,3
Based on the importance of evaluating the effectiveness of CAR-T, Creative Biolabs offers a variety of assays to help you achieve the therapeutic effect of evaluating CAR-T cell therapy.
| Brief introduction |
Non-specific imaging based on activated T cells: Based on antibodies and molecules that recognize proteins or pathways associated with activated T cells. This type of imaging platform targets: T cell activation markers, metabolic pathways, non-specific T cell antigens, or T cell immune checkpoints. Reporter-specific CAR-T cell imaging: Reporter genes are widely used in adoptive immunotherapy due to their high sensitivity and low toxicity. PET or SPECT-based ribonucleic acid imaging provides high resolution and sensitivity. |
| Brief introduction | Flow cytometry and qPCR are the most common methods for monitoring CAR-T cells in a clinical setting. Flow cytometry can assess the protein expression of CAR molecules on the cell surface, while quantitative PCR detects CAR vectors at the genomic level. Flow cytometry can not only assess the survival rate of CAR-T cells, but also perform multiparametric analysis of the immunophenotypic characteristics of these cells. |
| Published figure |
|
| Brief introduction | The pathological diagnosis process mainly includes four steps: sampling, preparation, diagnosis, and reporting. According to the type of sample, it can be divided into two categories: histological examination and cytological examination. Immunohistochemistry enables morphological and visual analysis of tumor cells and CAR-T cells from animal tissue sections such as bone marrow, spleen, periodontal region, and skull. |
| Brief introduction | Single-cell sequencing technology enables high-resolution molecular characterization of the activation and differentiation status of immune cells, so it has great potential for understanding CAR-T cell behavior. There is growing evidence that scRNA-seq technology can help reveal the correct receptor design selection, guide T cell modification and optimization of production conditions, which are necessary for successful therapeutic effects and long-term immune surveillance, and have the potential to be used as a means of clinical monitoring and efficacy prediction of CAR-T in the clinical treatment stage. |
| Brief introduction | Clinical observation involves characterization of the model, including metrics related to the research project, such as weight, behavior, and morphology. During the completed lifetime, all data are recorded and saved, and finally the corresponding statistical analysis is carried out. |
Creative Biolabs is a leading global supplier in the field of CAR-T. You can trust us with your project. Our team of experts is always in touch with you to communicate the progress of your project and ultimately deliver reliable, complete data results.
 Fig.3 Highlights of our service.
Fig.3 Highlights of our service.
Q1: What should I do if I want to inquire a CAR-T product efficacy test in Creative Biolabs?
A: You can contact our team of experts directly. If you have a complete testing method and process, you can ask for a quote directly. If not, you can send us a request, and we will provide you with a customized solution.
In addition to the above CAR-T efficacy tests, we also provide more CAR-T Preclinical In Vivo Assays, you can click directly to see more.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

