All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Traditional CAR-T cell therapies have revolutionized cancer treatment by engineering T cells to recognize and target cancer-specific antigens. However, these therapies have faced challenges related to safety, efficacy, and manufacturing. mRNA-based CAR technology has emerged as a promising alternative, offering several distinct advantages.
One of the key differentiators of mRNA-based CAR technology is its nonviral nature. Unlike traditional CAR-T therapies, which rely on viral vectors to deliver genetic material into T cells, mRNA-based CAR therapy utilizes modified mRNA molecules. These mRNA molecules are engineered to transiently express the CAR on the T cell surface, without permanently altering the host genome.
Within our laboratory, we have established a safer approach to cell therapy through the transient expression and non-viral delivery of one or more mRNA molecules into peripheral blood mononuclear cells (PBMCs) or isolated immune cells, including T cells or NK cells. Typically, we employ electroporation to introduce mRNA encoding CAR/TCR targeting specific tumor antigens into various cell lines. In comparison to cDNA, mRNA exhibits superior transfection efficiency while exerting minimal impact on cell viability.
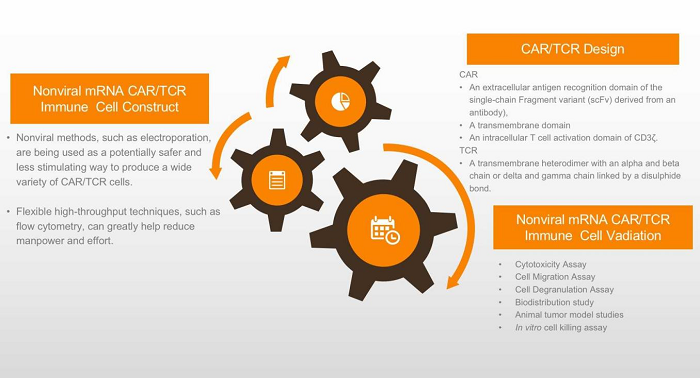 Fig. 1 The Workflow of Nonviral mRNA-Based CAR/TCR Immune Cell Platform.
Fig. 1 The Workflow of Nonviral mRNA-Based CAR/TCR Immune Cell Platform.
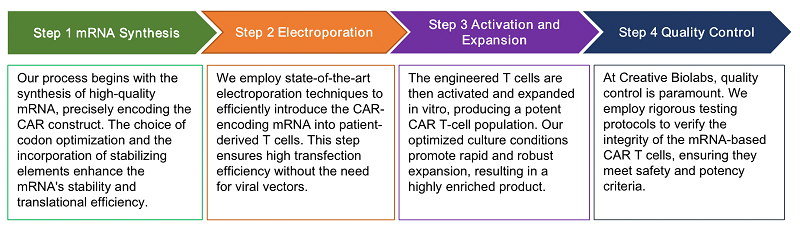
mRNA encoding anti-CD19 CAR was transfected into PBLs or PBMCs. Cells were cryopreserved and thawed for in vitro studies. The expression of CAR can be detected in vitro for about 6-10 days, at the same time, with the expansion of cells, the expression of CAR also gradually decreased. Furthermore, results in animal cancer models suggest that CAR mRNA can safely further control disease progression and prolong survival rate.
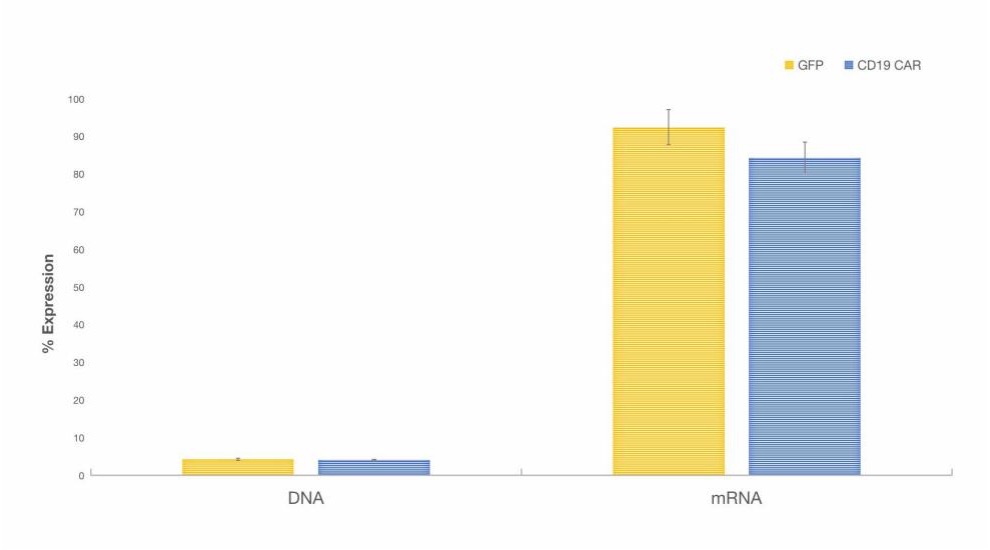 Fig. 3 Expression Levels of GFP or CD19-CAR Proteins in T Cells Electroporated with DNA or mRNA.
Fig. 3 Expression Levels of GFP or CD19-CAR Proteins in T Cells Electroporated with DNA or mRNA.
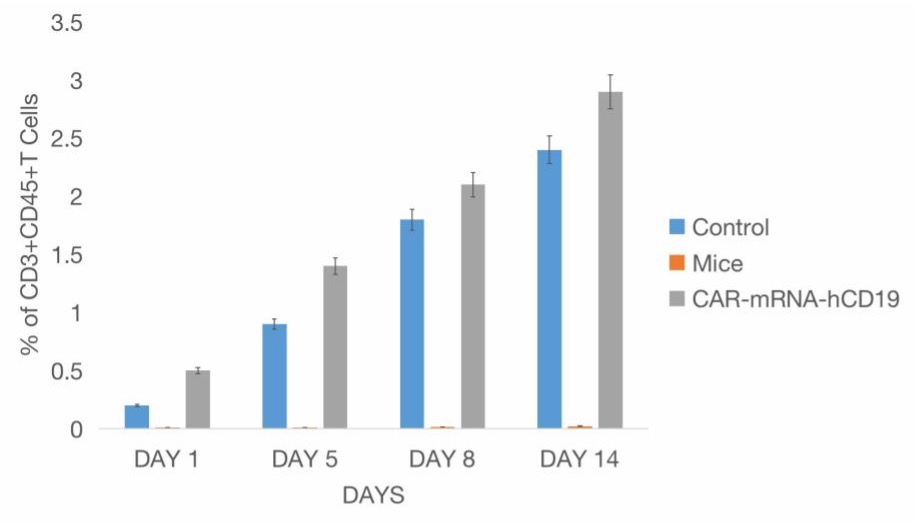 Fig. 4 Biodistribution Analysis of CAR-mRNA-hCD19 Cells Following Intraperitoneal (i.p.) Injection in a Mice Model.
Fig. 4 Biodistribution Analysis of CAR-mRNA-hCD19 Cells Following Intraperitoneal (i.p.) Injection in a Mice Model.
Creative Biolabs is specialized in designing and performing high-quality custom nonviral mRNA-based CAR/TCR cells analysis services, with different formats or parameters, to satisfy any specific requirement at the most competitive price.
For more detailed information, please feel free to contact us for more information and a detailed quote.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION