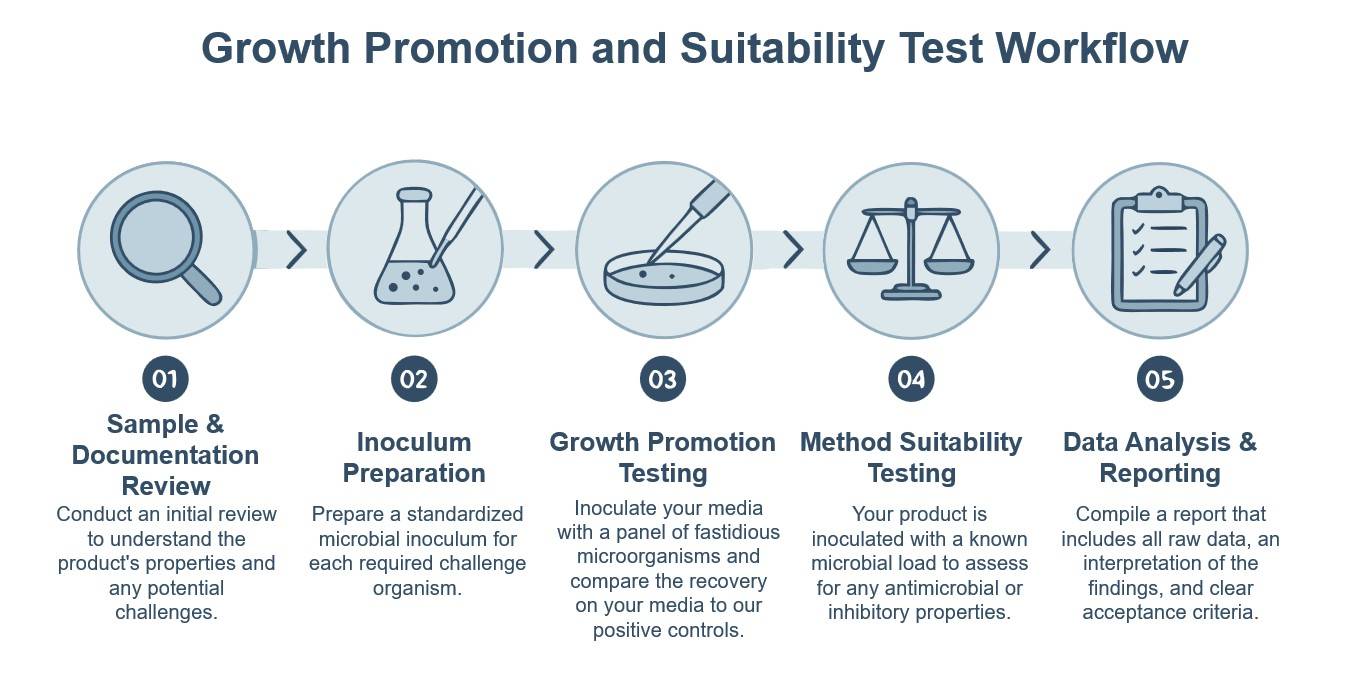
Growth Promotion & Suitability Test Service
Creative Biolabs provides a clear path to ensuring the reliability and compliance of your microbial quality control. We offer specific deliverables and problem-solving capabilities to mitigate risk and fortify your reputation. Our services are designed to provide objective, defensible data that confirms the integrity of your media and the validity of your test methods. You can expect a meticulous process that uncovers potential inhibitory effects from your product matrix, validates media fertility, and delivers detailed, auditable reports. This allows you to stand behind your product's safety with unwavering confidence.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
Introduction of Growth Promotion and Suitability Testing
Growth promotion and suitability testing are essential procedures in the domain of microbiological quality control. Growth promotion ensures that a specific batch of culture media is capable of supporting the proliferation of target microorganisms, whereas suitability testing verifies that the complete test methodology is not compromised by the presence of inhibitory substances within the product itself. Both tests are formally mandated by international pharmacopeias to preclude false-negative outcomes and ascertain the integrity of microbial assays. Ultimately, these protocols establish a rigorous scientific basis for microbial quality control, thereby reinforcing the safety and integrity of finished products.
To explore how our expertise can be leveraged to address the unique challenges of your project -Request a consultation.
What We Can Offer
Our service is a comprehensive offering that ensures the reliability of your microbiological testing. We provide the following key services to support your quality control needs.
|
Available Services
|
Description and Features
|
|
Growth Promotion Testing
|
We validate new batches of culture media to ensure they are capable of supporting microbial growth. This is a critical step to prevent false-negative results and comply with regulatory requirements.
|
|
Method Suitability Testing
|
We assess your product for any antimicrobial or inhibitory properties that could interfere with microbial testing. This ensures your final product can be accurately tested for microbial contamination.
|
|
Custom Microorganism Panels
|
We can tailor our testing to your specific needs, using a wide range of standard pharmacopoeial microorganisms or even your facility's own environmental isolates.
|
|
Neutralization Method Development
|
If your product proves to be inhibitory, we will develop and validate a method to neutralize its effects, allowing you to proceed with a reliable and compliant microbial assay.
|
Growth Promotion and Suitability Test Service at Creative Biolabs
Highlights
State-of-the-Art Facilities
We utilize cutting-edge laboratory equipment and follow validated, modern methodologies. This investment in technology allows us to provide highly accurate, reproducible results and handle a wide range of sample types and complexities.
Unwavering Partnership
We don't just perform tests; we provide a collaborative partnership. Our goal is to work with you to fortify your microbial quality control processes and build a foundation of trust for your products.
Uncompromising Accuracy
Our services are meticulously performed with an unwavering commitment to scientific accuracy. We ensure that every result is reliable and defensible, providing you with data you can confidently stand behind.
Tailored Solutions
Understanding that each client's needs are unique, we offer a flexible, consultative approach. We work closely with you to design a testing plan that directly addresses your specific project requirements, ensuring that the service is both efficient and effective.
To learn more about the advantages of partnering with Creative Biolabs and to receive a detailed service proposal, get a quote today.
Customer Reviews
-
Tangible Benefit
Creative Biolabs' growth promotion and suitability test service has significantly improved the reliability of our sterility testing research, providing dependable results and valuable insights for our ongoing projects. - J**s D.
-
Eliminated Problem
This service has eliminated all uncertainty from our media batches. The detailed reports give us a clear pass/fail, which is an invaluable resource for our laboratory's operational efficiency. - L**a W.
FAQs
How often should these tests be performed?
A growth promotion test is typically performed on every new lot or batch of prepared media. A suitability test, however, is generally a one-time validation for a new product or a significant change in its formulation or manufacturing process. We can help you define a testing schedule that meets your specific needs.
What if I need to test a microorganism not listed in the standard pharmacopeial panel?
Our services are highly flexible. We can work with you to test a wide range of microbial strains, including environmental isolates from your own facility. Our goal is to provide a tailored solution that directly addresses your unique microbial control challenges.
Related Services
Microbial Enumeration Service
Accurately quantify the microbial load in your product. This service is a fundamental step in ensuring product quality and safety, as it provides a clear picture of the microbial bioburden.
Learn More →
Sterility Testing Service
Our sterility testing service determines if your product is free of viable microorganisms. This is a critical final-step test for any product intended to be sterile, such as injectables, ophthalmics, and medical devices.
Learn More →
How to Contact Us
While routine microbiological testing is standard practice, the reliability of those results is directly dependent on the quality of the tests themselves. It is here that two critical, and often misunderstood, procedures come into play: the Growth Promotion Test and the Suitability Test. Our team is ready to answer your questions and help you design a testing strategy that ensures the safety and quality of your products. Please contact us.
For Research Use Only | Not For Clinical Use




