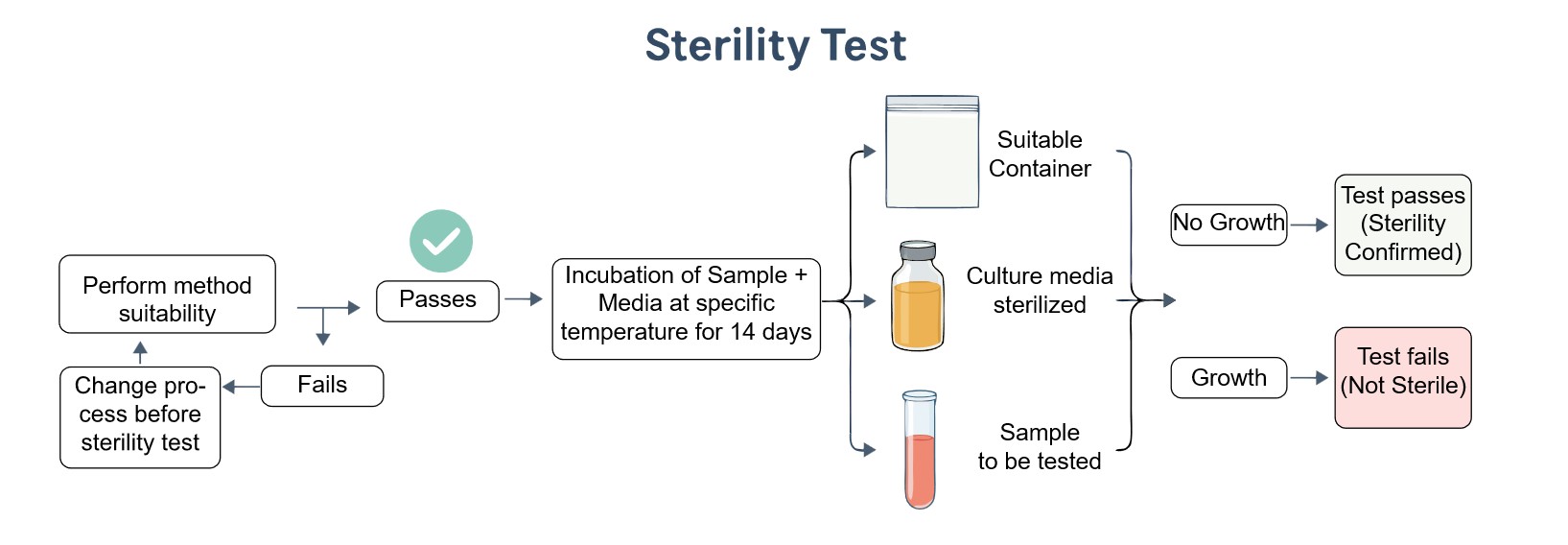
Sterility Test Service
Creative Biolabs' sterility test service provides a clear, compliant, and efficient methodology for confirming the absence of microbial contamination in products. This service generates a definitive "sterile" or "non-sterile" result, supported by a comprehensive report that documents each stage of the analysis. Our goal is to serve as a critical partner in your product's journey from development to market, providing the definitive assurance that your product is safe for its intended use.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
Advancing Sterility Testing Beyond Traditional Limitations
Sterility testing is a critical microbiological assay performed to confirm that a pharmaceutical product or medical device is free from viable microorganisms. This process is mandated by major global pharmacopeias to ensure product safety. Traditional methods, while reliable, often require a long incubation period. The development of advanced therapeutic products with short shelf lives has accelerated the need for rapid microbial methods (RMMs) and molecular techniques. These modern approaches, including ATP-bioluminescence and cytometry, provide faster, more accurate results, while advanced technologies like machine vision are being developed to automate the process and reduce human error.
For a detailed assessment of how our services can benefit your project, request a consultation.
What We Can Offer
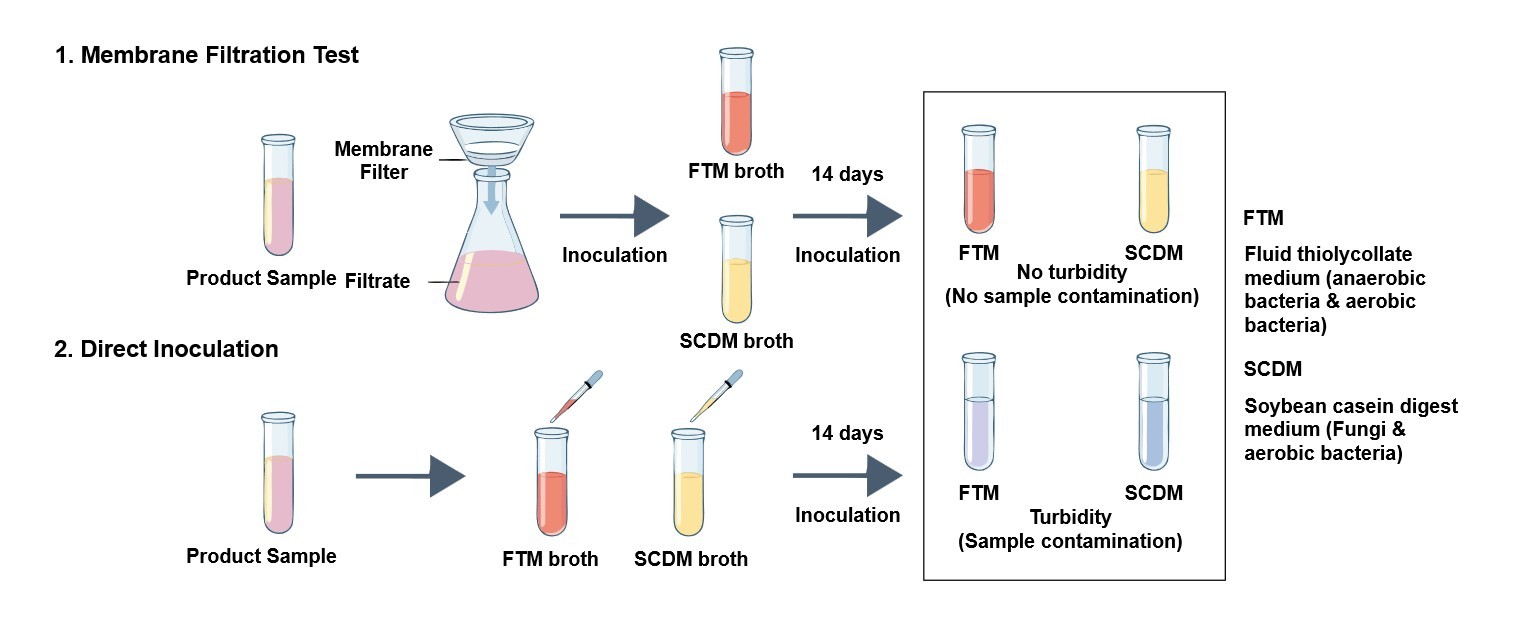
Membrane Filtration
This methodology is well-suited for filterable products, such as sterile liquids. The process involves passing the product through a size-exclusion membrane capable of retaining microorganisms, which are subsequently cultivated in an appropriate growth medium to facilitate the detection of contamination.
Direct Inoculation
This technique is employed for products that are not readily filterable, including medical devices or viscous materials. It entails the direct addition of the test material into the culture media to enable the observation of microbial growth over the designated incubation period.
Sterility Test Service at Creative Biolabs
Highlights
Experience and Expertise
Creative Biolabs is a partner with a deep scientific understanding of both traditional and modern methodologies. Our services are a testament to our extensive experience and knowledge in microbial testing and quality assurance.
Rapid Microbial Methods
We remain at the forefront of the industry by utilizing cutting-edge rapid microbial methods (RMMs). This dual capability allows us to provide a comprehensive solution that is tailored to your product's unique needs, particularly for time-sensitive materials.
Tailored Solutions
Our approach is not a one-size-fits-all model. We leverage our diverse expertise to provide solutions customized to your specific needs, whether you are developing traditional pharmaceuticals or cutting-edge cellular therapies.
Efficiency and Reliability
Our focus extends beyond mere compliance. We provide the most efficient and reliable path to market, ensuring your product is not only safe but also reaches consumers as quickly and reliably as possible.
To gain a comprehensive understanding of Creative Biolabs' capabilities, we invite you to get a quote today.
Customer Reviews
-
Streamlined Process
Their direct inoculation method handled our viscous material with ease, eliminating the need for complex sample dilution. This facilitated our in-house QC process and saved us a lot of time. - Prof. Dl Sh.
-
Proactive Quality Control
The suitability test was a crucial step we hadn't considered. It prevented a major issue for our product. This attention to detail has significantly improved our overall confidence in our manufacturing process. - Pl Ws.
FAQs
What if my product has antimicrobial properties?
This is a common and important concern. Our initial suitability test is designed specifically to address this. During this test, we confirm that our media can still support microbial growth even in the presence of your product, preventing false-negative results.
What is the difference between direct inoculation and membrane filtration?
The choice between these two methods depends on your product. Membrane filtration is ideal for large volumes of filterable liquids and is particularly useful for products with inhibitory substances. Direct inoculation is the method of choice for products that are not easily filtered, such as viscous liquids or certain medical devices.
Related Services
Antimicrobial Effectiveness Test (AET)
Assess the efficacy of preservatives in non-sterile products by challenging them with specific microorganisms. This service ensures your product's preservative system is robust and compliant with pharmacopeial standards.
Learn More →
Antimicrobial Susceptibility Testing (AST)
Assess the effectiveness of your antimicrobial agents against a panel of microorganisms. This service helps determine the optimal dosage and spectrum of activity, ensuring your product's antimicrobial claims are supported by robust data.
Learn More →
How to Contact Us
Creative Biolabs is committed to providing the highest level of scientific expertise and customer service. Our team of specialists is ready to discuss your specific project needs and help you find the right solution. To learn more about our sterility test service and how we can assist with your specific project, please contact us.
For Research Use Only | Not For Clinical Use





