Liposome Development Service for Enhanced Sonosensitizer Delivery
Background Advantages Workflow Publication Why Choose Us FAQs Customer Review Related Services Contact Us
Revolutionizing Sonodynamic Therapy with Advanced Liposome-Based Drug Delivery
Liposomes, drug-encapsulating lipid vesicles, are transforming drug delivery by enhancing solubility, reducing toxicity, and enabling targeted action. Sonodynamic therapy (SDT) uses ultrasound-activated sonosensitizers to generate reactive oxygen species, selectively destroying cancer cells. Our service at Creative Biolabs utilizes advanced liposome technology to overcome sonosensitizer delivery challenges, improving tumor specificity and therapeutic efficacy. We offer custom-designed, stable, and efficiently loaded liposomal formulations that ensure enhanced tumor accumulation and controlled, triggered release of your active agent. This approach significantly reduces systemic toxicity, improves the therapeutic index, and provides comprehensive characterization data, along with scalable manufacturing processes for clinical readiness.
Why Liposomes Are the Ideal Vehicle for Sonosensitizer Delivery
Exceptional Biocompatibility and Biodegradability
Made from natural phospholipids, liposomes are highly biocompatible, ensuring low immunogenicity and excellent tolerability.
Versatile Encapsulation
They efficiently encapsulate both water-soluble and lipid-soluble sonosensitizers.
Enhanced Pharmacokinetics and Bio-distribution
Liposomes accumulate in tumors via the enhanced permeability and retention (EPR) effect. PEGylated liposomes evade the immune system, extending circulation time.
Controlled and Triggered Release
Advanced designs allow for precise sonosensitizer release in response to tumor-specific triggers like pH, temperature, or ultrasound.
Precision Targeting
Surface functionalization with ligands enables active targeting of cancer cells, minimizing off-target effects.
Protection and Stability Enhancement
Liposomes protect sonosensitizers from degradation, improving stability and preserving activity.
Request a Quote
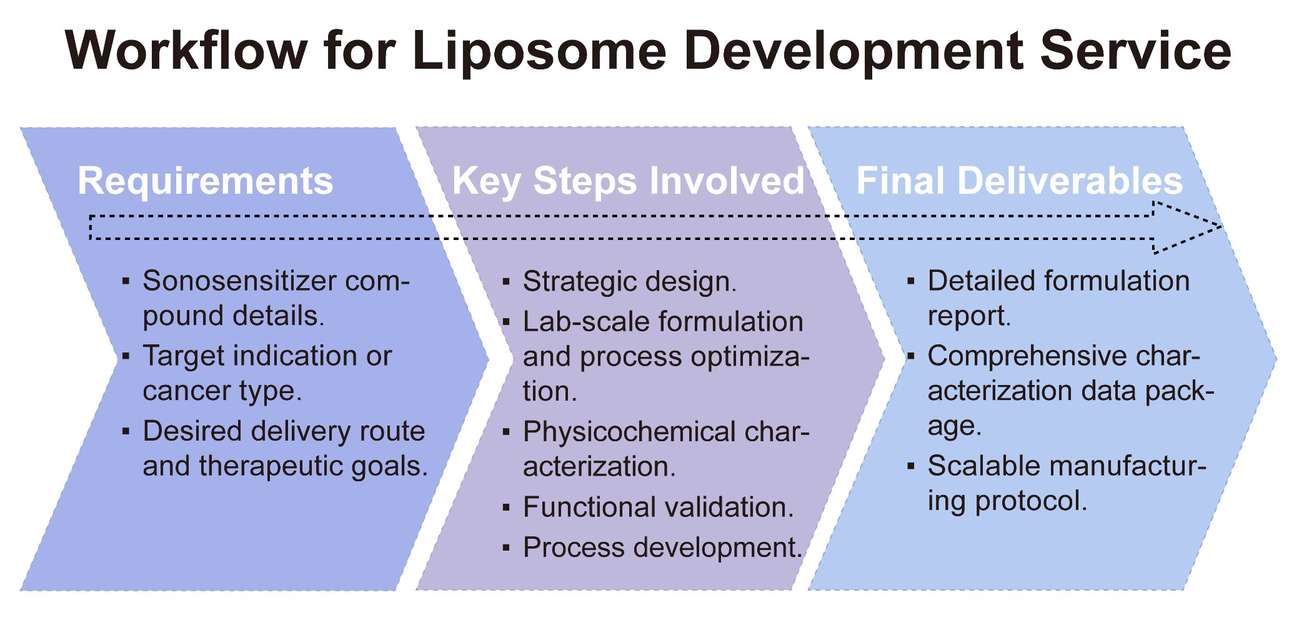
Workflow for Sonosensitizer Liposome Development
Developing a successful liposome-based sonosensitizer delivery system is a multi-faceted process that demands meticulous planning and execution. Our streamlined workflow ensures clarity, efficiency, and consistent quality at every stage, making the journey from concept to clinic as smooth as possible.
Publication
This publication reviews the evolution of liposome-based drug delivery systems from initial laboratory models to their current status as clinically approved therapeutics. Liposomes are versatile, spherical vesicles composed of phospholipid bilayers that can encapsulate various drugs, addressing pharmacokinetic issues like poor solubility and systemic toxicity. The review highlights their structural adaptability, functional tunability, and transformative impact on modern medicine, discussing laboratory-scale preparation techniques, industrial production challenges, and innovative strategies like microfluidic systems and advanced process optimization to overcome these hurdles. Furthermore, the paper explores emerging trends including stimuli-responsive and hybrid liposomes, and their integration with nanotechnology for enhanced therapeutic precision, emphasizing their potential to redefine pharmaceutical applications in areas like gene therapy, theranostics, and personalized medicine. Despite existing challenges, continuous advancements in formulation and scalability suggest a promising future for liposome-based therapeutics in addressing unmet medical needs.
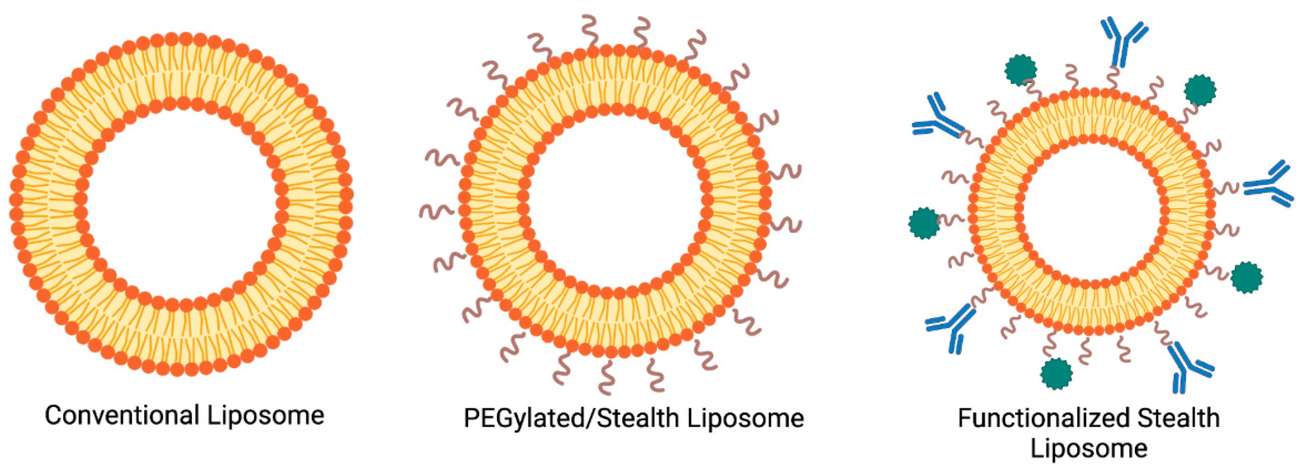 Fig.1 Adjustable structural and functional properties of standard liposomes.1
Fig.1 Adjustable structural and functional properties of standard liposomes.1
Why Choose Us?
At Creative Biolabs, we leverage over a decade of experience in lipid-based nanocarriers to offer unparalleled, end-to-end liposome development. Our unrivaled scientific expertise and cutting-edge technology, including microfluidic platforms, ensure precise, reproducible, and scalable manufacturing. We specialize in stimuli-responsive liposome design, particularly ultrasound-responsive formulations for localized release. With comprehensive characterization, we ensure clinical readiness. Our advanced active targeting capabilities further enhance tumor specificity and therapeutic efficacy, delivering robust, high-quality solutions for your sonosensitizer projects.
FAQs
Q1: What types of sonosensitizers can be encapsulated by your service?
A1: We have extensive experience encapsulating a wide range of sonosensitizers, including both hydrophilic and hydrophobic compounds. Our versatile methods are tailored to the unique properties of your specific agent to achieve optimal loading efficiency and maintain its integrity.
Q2: How do you ensure the stability and shelf-life of the developed liposomal formulations?
A2: Stability is paramount for clinical translation. We use rigorous optimization, cryoprotectants, and antioxidants to protect liposomes. Comprehensive stability studies and lyophilization services ensure long-term shelf life, making your product practical for storage and distribution.
Q3: What is the typical turnaround time for a liposome development project, and what factors influence it?
A3: Project timelines vary with complexity. Factors include sonosensitizer novelty, formulation optimization, and in vivo studies. We ensure efficient project management to accelerate your progress without compromising quality.
Customer Review
-
Improved Encapsulation
Creative Biolabs successfully solved a problem with low encapsulation efficiency for a hydrophilic sonosensitizer. Using active loading techniques, they achieved over 90% encapsulation, which was crucial for reaching therapeutic concentrations. - Dr. S***n B
-
Overcoming Aggregation
Creative Biolabs solved our liposome aggregation issue by optimizing our formulation with specific charged lipids and cryoprotectants. Their expertise in lyophilization provided a stable, reconstitutable product with an extended shelf-life, saving significant time and resources. - Dr. P***r R
Related Services
To further support your drug development journey, Creative Biolabs offers a suite of complementary services and specialized variations of our liposome development service:
Manufacturing for Clinical Trials
We ensure seamless transition from development to large-scale production of your liposomal sonosensitizer. Our expertise guarantees quality and consistency for clinical readiness, accelerating your path forward.
Learn More →
Toxicology and Safety Assessment
Comprehensive in vitro and in vivo toxicology studies to ensure the safety profile of your liposomal formulation, essential for regulatory submissions.
Learn More →
How to Contact Us
At Creative Biolabs, we are dedicated to empowering your research and development efforts with our unparalleled expertise in liposome technology. Our liposome development service for is designed to overcome complex challenges and accelerate your path to clinical success.
Contact Us for Project Details.
Reference
-
Suman, Basak, and Tushar Kanti Das. "Liposome-Based Drug Delivery Systems: From Laboratory Research to Industrial Production—Instruments and Challenges." ChemEngineering 9.3 (2025): 56. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3390/chemengineering9030056



 Fig.1 Adjustable structural and functional properties of standard liposomes.1
Fig.1 Adjustable structural and functional properties of standard liposomes.1
