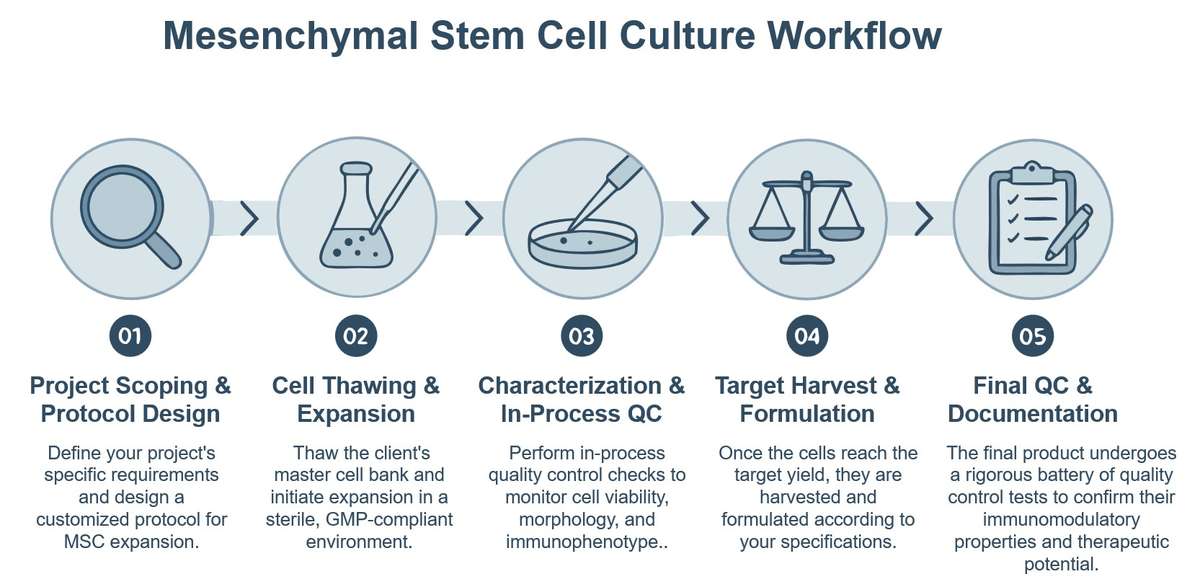
Mesenchymal Stem Cell Culture Service for ATMP Development
Creative Biolabs provides a comprehensive and de-risked solution for the development of clinical-grade mesenchymal stem cells (MSCs), thereby facilitating a seamless transition from research-scale to commercial-scale manufacturing. By managing the complexities inherent in cell culture, including scale-up challenges and the need for consistent product quality, we deliver tangible results that enable clients to concentrate on their core R&D and clinical trial objectives. This strategic partnership allows your team to redirect resources toward critical upstream and downstream activities.
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
Advanced MSC Culture for Enhanced ATMP Therapies
The cultivation of MSCs constitutes a critical and foundational process for the development of advanced therapeutic medicinal products (ATMPs), which serve as the basis for regenerative and immunomodulatory therapies. This process encompasses the meticulous isolation, controlled expansion, and comprehensive characterization of these multipotent cells within a highly regulated environment. This approach is substantiated by decades of scientific research and a substantial body of published literature demonstrating the advantages of advanced techniques for enhancing therapeutic efficacy.
Request a consultation to discuss project requirements.
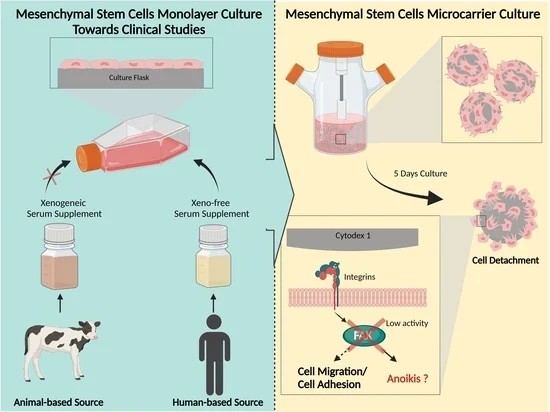 Fig.1 Enhancing MSC culture: 3D microcarriers and anoikis resistance. 1
Fig.1 Enhancing MSC culture: 3D microcarriers and anoikis resistance. 1
What We Can Offer
Standardized Isolation and Expansion
We have established, validated, and standardized processes for isolating and expanding MSCs from a variety of tissue sources, including bone marrow, adipose tissue, and umbilical cord. This guarantees a consistent and high-quality starting material for every project, regardless of origin.
Scaled Manufacturing
Our facilities are equipped for scaled manufacturing using a variety of advanced bioreactor systems. We can seamlessly scale from pilot-batch to full-scale production, ensuring the consistency and viability of your cells across thousands of doses.
Comprehensive Characterization
Our extensive characterization package is fully aligned with the international society for cell therapy (ISCT) criteria. We provide in-depth analysis of immunophenotype, tri-lineage differentiation potential, and other critical markers to ensure your cell product meets scientific standards.
Specialized Formulation
We go beyond simple cell expansion by offering specialized formulation services for improved stability and post-thaw recovery. Our methods are designed to maximize cell viability and efficacy for your application, ensuring your therapeutic product remains potent.
Mesenchymal Stem Cell Culture Service at Creative Biolabs
Highlights
Strategic Partnership
A strategic partnership with Creative Biolabs is a foundational element for ATMP development. This collaboration offers a competitive advantage rooted in a profound scientific foundation and a dedicated focus on mutual success, transcending the typical client-vendor relationship.
Unparalleled Scalability
The platform provides unparalleled scalability for projects. This capability facilitates a seamless transition from initial research to full-scale production, ensuring that product quality and operational efficiency are maintained at every stage of development.
Advanced Culture Techniques
The service employs advanced techniques, which have been demonstrated to enhance the therapeutic efficacy of MSCs by improving their immunomodulatory properties and engraftment efficiency.
Relentless Quality Focus
A defining characteristic of this approach is a persistent focus on quality. Every batch of MSCs is ensured to meet the most stringent industry standards, thereby providing the consistency and reliability essential for a robust development pipeline.
For a detailed proposal, we invite you to get a quote today.
Customer Reviews
-
Improved Consistency & Yield
Using Creative Biolabs' service in our research has significantly improved the consistency and yield of our cell expansion. The detailed documentation provided with each batch has been a huge asset for our regulatory submissions. - Rd Pk, Senior Research Scientist.
-
Exceptional Scientific Support
The level of scientific support from Creative Biolabs has been exceptional. They helped us troubleshoot a persistent contamination issue and provided a closed-system solution that eliminated the problem. - Mn Lz, Director of Process Development.
FAQs
How do you ensure the quality and consistency of the manufactured cells?
We follow a comprehensive quality control program based on ISCT guidelines. This includes extensive in-process checks and final-product testing for immunophenotype, viability, sterility, and potency. Our use of closed-system bioreactors further ensures batch-to-batch consistency and minimizes the risk of contamination.
What are the benefits of using a 3D culture system over traditional 2D culture?
Published data show that 3D culture systems can enhance the therapeutic properties of MSCs, including improved immunomodulatory function and engraftment efficiency. We have the expertise to utilize these advanced systems to create a more potent and effective final product.
Related Services
MSC Differentiation Services
We provide specialized protocols and optimized media to induce the differentiation of MSCs into specific lineages, such as osteocytes, chondrocytes, and adipocytes, for targeted research and therapeutic applications.
Learn More →
Immunomodulation Analysis
We offer advanced assays to analyze the immunomodulatory effects of your MSCs, including their interaction with immune cells and the effects of secreted cytokines. This is essential for developing therapies for conditions like GvHD.
Learn More →
How to Contact Us
Creative Biolabs is ready to partner with you to accelerate your ATMP development. Our team of experts is committed to providing the dedicated support and innovative solutions you need to navigate the complexities of this field and achieve your therapeutic goals. For more information and to discuss your project, please contact us.
Reference
-
Koh, Benson et al. "A Three-Dimensional Xeno-Free Culture Condition for Wharton's Jelly-Mesenchymal Stem Cells: The Pros and Cons." International journal of molecular sciences vol. 24,4 3745. 13 Feb. 2023. Distributed under an Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms24043745
For Research Use Only | Not For Clinical Use


 Fig.1 Enhancing MSC culture: 3D microcarriers and anoikis resistance. 1
Fig.1 Enhancing MSC culture: 3D microcarriers and anoikis resistance. 1


