Creative Biolabs offers unique and unrivaled expertise in the research, development,
validation and
multi-gram manufacture of therapeutic antibodies and proteins. Our scientific knowledge provides the full
process of the mAb project from early Purification Development to successful manufacturing.
Application Prospect
Over the decades, the therapeutic antibody has been successfully introduced into drug libraries for treating
patients, with fierce innovation in antibody production and engineering. Antibody engineering involves the
introduction of antibody binding sites (variable regions) into a series of structures that further influence
treatment characteristics, thus achieving further advantages and success in patient treatment.
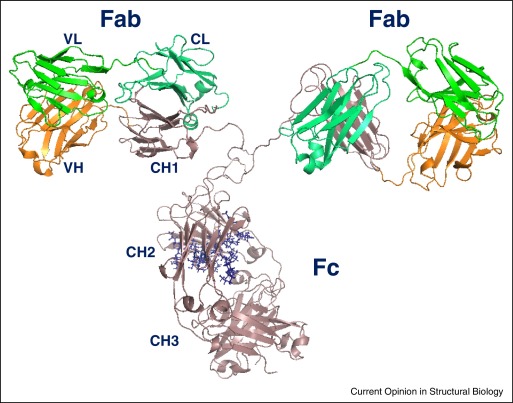 Fig.1 Structural model of a human IgG1.1
Fig.1 Structural model of a human IgG1.1
Our experienced team can effectively shorten the clinical batch time of the monoclonal antibody development
project. The service modules we provide cover the whole process from antibody discovery to development and
optimization.
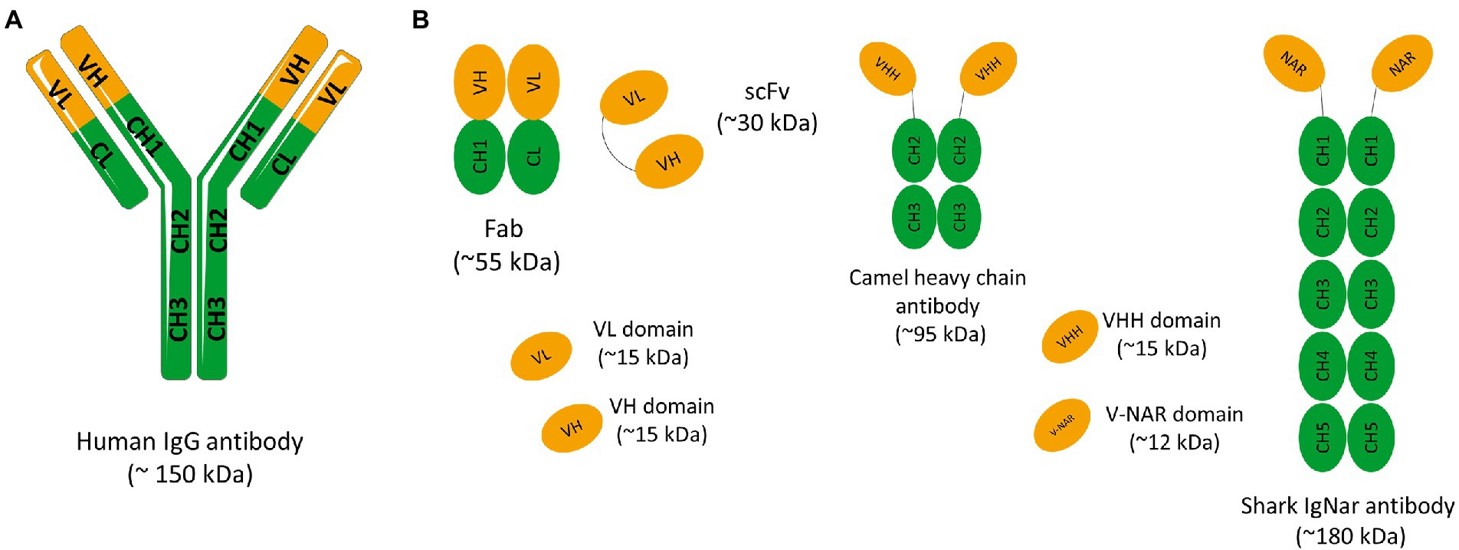 Fig.1 Schematic representation of a conventional IgG antibody.2
Fig.1 Schematic representation of a conventional IgG antibody.2
Process Overview:
Creative Biolabs provides custom selection conditions to achieve the highest
specificity and to address complex issues such as difficult target antigens or
human/rat cross-reactions. We provide the following antibody customization
services, but not limited to these:
Prominent Feature
Based on our comprehensive range of capabilities, we are able to meet all of your mAb process development
requirements:
-
We offer an efficient transient transfection platform: HEK and CHO cells, ideal for proof-of-concept and
antibody screening programmes.
-
We provide comprehensive monoclonal and polyclonal antibody development services in the fields of
antigen design, peptide synthesis and recombinant protein production.
-
Searching service for the sequence or accession number of the protein
-
Offering the best possible epitope for antibody generation.
-
Our antibody process development service fits customer demands for fast-track process development.
Tell us what you need and we will provide a guide to project cost estimates and timelines as soon as possible so
that you can plan your next steps after the service is completed. Contact us or send us a query directly.
References
-
Chiu, Mark L., and Gary L. Gilliland. "Engineering antibody therapeutics." Current opinion in structural biology 38 (2016): 163-173.
-
André, Ana S., et al. "In vivo Phage Display: A promising selection strategy for the improvement of antibody targeting and drug delivery properties." Frontiers in Microbiology 13 (2022): 962124.


 Fig.1 Structural model of a human IgG1.1
Fig.1 Structural model of a human IgG1.1
 Fig.1 Schematic representation of a conventional IgG antibody.2
Fig.1 Schematic representation of a conventional IgG antibody.2 Download our brochure
Download our brochure