Morphological Integrity in iPSCs
Morphology is the first and most intuitive indicator of iPSC quality. Subtle changes in colony appearance often precede detectable shifts in marker expression or functional behavior. Systematic morphology evaluation is therefore essential for:
-
Ensuring pluripotency and self-renewal
High-quality iPSCs show compact, high–nucleus-to-cytoplasm ratio cells with well-defined colony borders. Deviations can indicate loss of pluripotency or inappropriate differentiation. -
Detecting early differentiation and culture stress
Irregular edges, flattened cells, heterogeneous colony density, or mixed cell populations suggest suboptimal culture conditions, genetic instability, or stress responses that may compromise downstream differentiation outcomes. -
Evaluating genome-edited and disease-specific iPSC lines
Morphology analysis provides a straightforward way to compare edited vs. parental lines and monitor stability over passages. -
Standardizing raw materials for iPSC-derived models and assays
For projects involving drug screening, toxicity assays, organoid generation, or cell-based functional studies, researchers need consistent iPSC starting material. Morphological QC is a key element of robust batch-to-batch consistency. -
Supporting comprehensive iPSC characterization packages
When combined with karyotyping, pluripotency marker assays, qPCR, and functional tests, morphology analysis strengthens your documentation package for collaborations, publications, and preclinical studies.
Therefore, we have engineered a state-of-the-art iPSC Morphology Analysis Service. This service provides researchers and biomanufacturers with quantitative, unbiased, and highly scalable metrics.
Service Portfolio for iPSC Morphology Analysis
We offer a modular portfolio that can be tailored to your project stage and regulatory needs.
Table 1 Our service portfolio at a glance
| Services | Descriptions |
|---|---|
| Routine Colony Morphology Assessment |
|
| High-Content Imaging and Semi-Quantitative Scoring |
|
| Image-Based Quantification and Automated Analysis |
|
| Morphology Monitoring During Reprogramming and Early Expansion |
|
| Morphology Analysis Combined with Marker Staining |
|
Our services span the entire stem cell workflow, providing critical checkpoints for quality.
- Reprogramming & Clone Selection: Assessing early morphological transition, evaluating the efficiency of colony formation, and aiding in the selection of bona fide pluripotent clones.
- Routine Maintenance & Expansion: Non-invasive, daily or weekly QC monitoring to verify colony health, self-renewal capacity, and the absence of spontaneous differentiation or senescence, which are crucial for maintaining banking quality.
- Differentiation Readiness: Confirming the optimal morphological state (maturity and density) of the pluripotent starting material immediately prior to induced differentiation.
- Directed Differentiation Tracking: Monitoring morphological changes as iPSCs transition into progenitor and mature cell types (e.g., neurons, cardiomyocytes, hepatocytes), ensuring proper lineage commitment and maturity.
Our iPSC Morphology Analysis Service Highlights
- Dedicated stem cell imaging platform with phase - contrast, brightfield, and optional fluorescence.
- Standardized scoring criteria for colony shape, border definition, cell density, and differentiation signs.
- Quantitative metrics (e.g., colony size distribution, confluency, heterogeneity indices) available upon request.
- Integration with other iPSC QC assays (e.g., pluripotency markers, karyotype analysis, teratoma assays, EB characterization).
- Flexible sample formats - we can work with your iPSC lines, or with iPSCs generated and maintained on our in-house platform.
- We routinely adjust inputs for high-efficiency workflows; tell us your constraints.
Typical Applications of iPSC Morphology Analysis
Our iPSC Morphology Analysis Services support a wide spectrum of research and development activities, including but not limited to:
| Applications | Descriptions |
|---|---|
| Pre-screening of iPSC lines prior to differentiation | Identify the best batches and passage windows for generating neural, cardiac, hepatic, or immune cells. |
| Quality control for genome-edited or disease-specific iPSCs | Assess whether new edits or disease mutations alter colony morphology, growth characteristics, or culture stability. |
| Standardization for high-throughput screening | Ensure consistent starting material for drug screening, toxicity testing, or phenotypic assays, reducing variability and improving data reliability. |
| Support for organoid and 3D model generation | Confirm that precursor iPSC cultures meet quality criteria before embarking on more complex organoid or organ-on-chip workflows. |
Published Data
In a study, the researchers extracted and quantitatively analyzed seven morphological parameters of cells and colonies from three hPSC lines and linked these data to their clonality, pluripotency status, and differentiation ability towards three germ layers. They identified specific morphological parameters as the most informative ones in terms of variance between lines and different morphological features and used these parameters to train classification models of colony phenotypes.
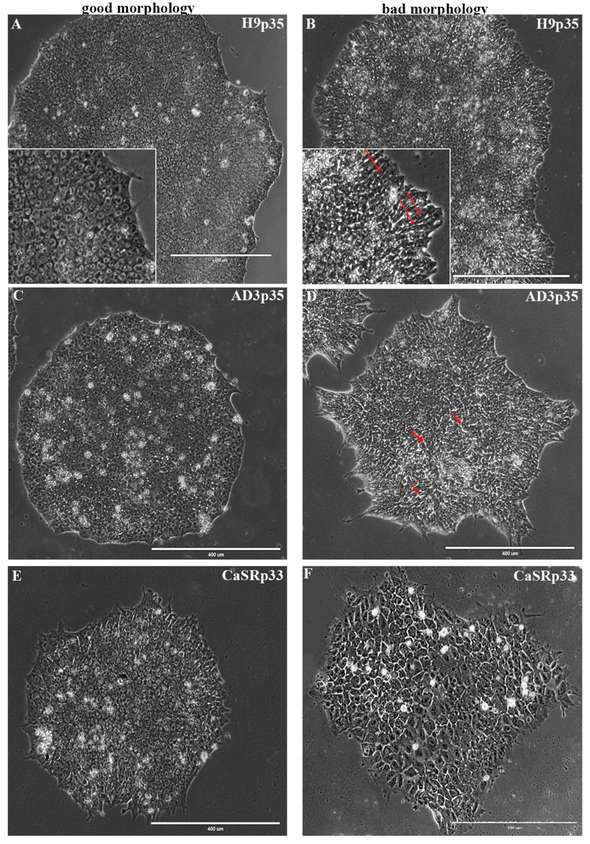 Fig. 1 Morphological features of hPSC colonies with good and bad phenotypes at 120 h after plating.1,3
Fig. 1 Morphological features of hPSC colonies with good and bad phenotypes at 120 h after plating.1,3
Using image analysis and computational tools, the researchers precisely quantify these properties using phase-contrast images of hESC colonies of different sizes during days 2, 3 and 4 after plating. Their analyses reveal noticeable differences in their structure influenced directly by the colony area.
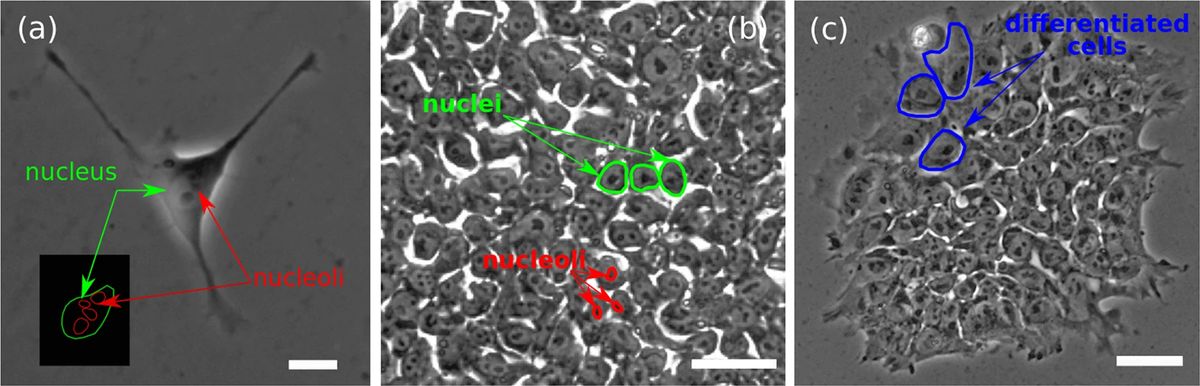 Fig. 2 The hESCs colonies were imaged.2,3
Fig. 2 The hESCs colonies were imaged.2,3
What Our Clients Say
"Their morphology reporting is meticulous, easy to understand, and incredibly actionable. Creative Biolabs consistently identifies early issues and provides concrete recommendations that help us maintain stable lines across long-term culture."
— Dr. M. Pereira, Senior Research Scientist
"We operate across three global research centers and needed a consistent third-party lab to evaluate morphology and culture stability. Creative Biolabs' standardized scoring system helps us compare data across sites and time points. Their communication is prompt, and reports arrive exactly when we need them."
— Prof. L. Nguyen, Principal Investigator
"Our disease-modeling program relies heavily on iPSCs with fragile phenotypes. Creative Biolabs' morphology assessments have repeatedly helped us stabilize culture conditions, refine passage timelines, and avoid early differentiation. Their recommendations have directly improved reproducibility in our assays."
— Dr. E. Vasilev, Group Leader, Molecular Neuroscience Laboratory
"One of our rare disease iPSC lines consistently displayed unpredictable growth patterns. Creative Biolabs conducted extended morphological tracking and identified culture stress patterns related to media changes. With their guidance, we resolved the instability and resumed differentiation successfully."
— S. Meyer, Research Associate, Gene Editing Program
FAQs
Q: How do you define a "high-quality" iPSC colony during morphology assessment?
A: A high-quality iPSC colony typically shows tightly packed cells, high nucleus-to-cytoplasm ratio, smooth colony borders, and uniform cell density. We evaluate these parameters using standardized scoring criteria and compare them with reference morphology from well-established pluripotent lines to determine overall colony quality and culture stability.
Q: Can you evaluate multiple passages to identify the optimal passage window for differentiation?
Q: What happens if my iPSC line shows early signs of spontaneous differentiation?
Q: What imaging modalities do you use for morphology analysis?
Q: Do you accept cryopreserved iPSC vials for analysis?
Q: How quickly can I receive the morphology analysis report?
Q: Can morphology analysis be integrated with other iPSC QC assays offered by Creative Biolabs?
Start Your iPSC Morphology Analysis Project














1. Contact Us
via the Inquiry Form or Email
2. Define Your Needs
Cell Type, Function, Quantity, Modifications
3. Kickstart the Project
Our Expert Team Guiding Every Step
Whether you are validating a new iPSC line, troubleshooting inconsistent differentiation outcomes, or setting up a robust stem cell manufacturing workflow, Creative Biolabs is ready to support you with reliable, high-quality iPSC Morphology Analysis Services.
Contact us today with your project details—our experts will help you design an efficient, tailored analysis plan that fits your timeline, budget, and scientific goals.
References
- Krasnova, Olga A., et al. "Prognostic analysis of human pluripotent stem cells based on their morphological portrait and expression of pluripotent markers." International Journal of Molecular Sciences 23.21 (2022): 12902. https://doi.org/10.3390/ijms232112902
- Orozco-Fuentes, Sirio, et al. "Quantification of the morphological characteristics of hESC colonies." Scientific Reports 9.1 (2019): 17569. https://doi.org/10.1038/s41598-019-53719-9
- Distributed under Open Access license CC BY 4.0, without modification.
Created November 2025

