Antibody Screening & Characterization Service
Background What We Can Offer Workflow Publication Why Choose Us FAQs Customer Review Related Services Contact Us
The Cornerstone of Modern Biologics Discovery
Monoclonal antibodies (mAbs) are rapidly expanding biopharmaceuticals revolutionizing disease treatments. Their precise interaction with targets is crucial for therapeutic success. Antibody screening and characterization are fundamental to ensure pure, consistent, stable, and safe antibody lots. This requires meticulous profiling of structure, post-translational modifications, and biomolecular/cellular activities using state-of-the-art analytical techniques. Creative Biolabs provides end-to-end solutions for antibody discovery and development, delivering high-quality, actionable data and lead candidates that meet specific therapeutic or diagnostic objectives. Their comprehensive approach vets every antibody, minimizing risks and accelerating success.
Detailed Antibody Screening and Characterization
|
Key Antibody Screening
|
Key Antibody Characterization
|
-
Primary Screening (10,000-50,000 samples per assay)
-
Screening by ELISA (protein-based)
-
Screening by FACS (cell-based)
-
Image-based homology assay (protein or cell-based)
-
Secondary screening (1,000-3,000 samples per assay)
-
Cross-species and cross-family screening
-
Quantitative ELISA / FACS
-
Competitive ELISA
|
-
Epitope Binding: Grouping antibodies according to the target area
they recognize.
-
Affinity Ranking: Relative affinity is selected by HTS.
-
Cross-reactivity: Negative screening for cross-reactivity is used to
guide the selection of appropriate specific clones.
-
Cell-based Screening: Antibodies against cell surface antigens are
the best screening in the native context.
|
Workflow: From Concept to Candidate
Our workflow is meticulously designed for clarity and efficiency, ensuring a seamless journey from your initial project concept to the delivery of well-characterized antibody candidates. Each stage is optimized to provide critical insights and advance your project with confidence.
Required Starting Materials:
-
Target antigen: Purified protein, cell lines with target expression, or DNA/RNA for in situ expression. High-quality antigen is crucial.
-
Project goals: Detailed objectives: desired affinity, specificity, and therapeutic application.
-
Previous data: Existing binding data, preliminary screens, or relevant background on target/similar antibodies to optimize screening.
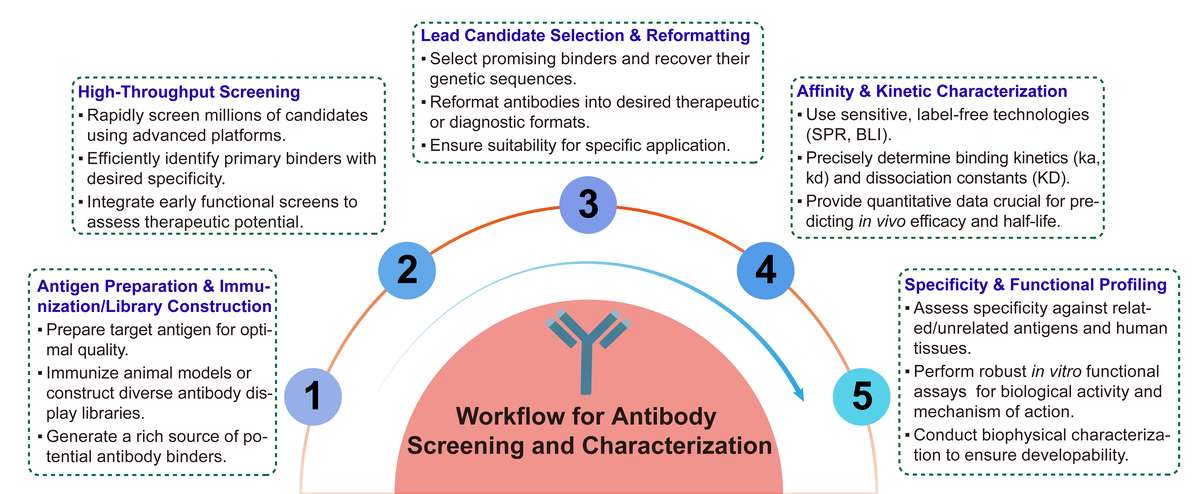
Final Deliverables:
-
Comprehensive data reports: Detailed reports with raw data, analyzed results, and expert interpretations for all assays, supporting regulatory submissions.
-
Lead candidate sequences: Verified heavy and light chain variable region sequences of selected antibodies.
-
Purified antibody samples: Gram-scale quantities of highly purified lead antibodies, ready for preclinical studies or manufacturing.
Publication
This publication reviews the major analytical techniques used for the comprehensive characterization and quantification of mAbs. mAbs are a rapidly growing class of biopharmaceuticals crucial for diagnostics and therapeutics. The successful application of therapeutic mAbs depends on their precise interaction with target sites, which necessitates detailed characterization of their structure, post-translational modifications, and activities at the biomolecular and cellular levels. The review summarizes chromatographic, electrophoretic, spectroscopic, and electrochemical methods, and their modifications, used to ensure the purity, consistency, stability, and safety of mAbs in accordance with regulatory guidelines. This compilation aims to provide researchers with an overview of methodologies employed in the biopharmaceutical industry for structural characterization of mAbs for drug development.
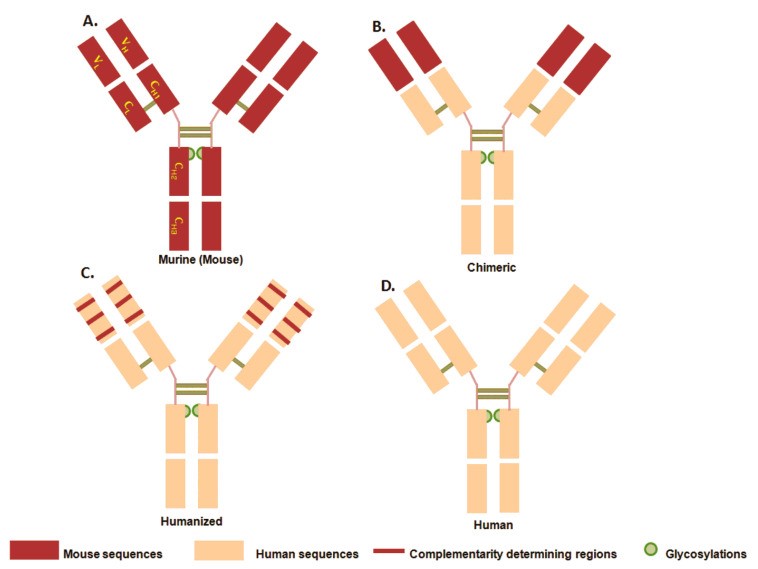 Fig.1 Four evolutionary stages of mAb.1
Fig.1 Four evolutionary stages of mAb.1
Why Choose Us?
Creative Biolabs is a leading force in antibody discovery, boasting over 10 years of experience and a relentless pursuit of scientific innovation. Their commitment to quality, efficiency, and client success distinguishes them in the field, ensuring productive and successful antibody development journeys. They leverage extensive expertise and cutting-edge technologies to deliver high-quality, actionable data and lead candidates that precisely meet therapeutic or diagnostic objectives. By minimizing risks and accelerating timelines, Creative Biolabs is dedicated to transforming research challenges into tangible breakthroughs for diverse applications.
Our Strengths:
-
Guaranteed success: Each step of your project is backed by a success-based guarantee.
-
Comprehensive solutions: We offer a complete and flexible pipeline with a wide range of options.
-
State-of-the-art equipment: Your project benefits from the best and most efficient equipment.
-
Dedicated to excellence: Our approach ensures high-quality outcomes for your project.
Experience the Creative Biolabs Advantage - Get a Quote Today
FAQs
Q1: What is the typical purity of the antibody samples delivered?
A1: Creative Biolabs aims for industry-leading purity levels, typically greater than 95% for lead antibody candidates, and often exceeding 98% for final purified samples, as assessed by techniques like SDS-PAGE and SEC-HPLC. We ensure the purity is suitable for your intended downstream applications, including in vivo studies.
Q2: Do you offer services for both therapeutic and diagnostic antibody development?
A2: Yes, Creative Biolabs provides comprehensive Antibody Screening and Characterization services for both therapeutic and diagnostic applications. Our platforms are flexible and can be tailored to meet the unique requirements of each, whether you need high-affinity binders for in vivo treatment or highly specific reagents for in vitro diagnostic assays.
Q3: Can you assist with post-translational modification analysis of the antibodies?
A3: Absolutely. Comprehensive analysis of post-translational modifications (PTMs) is a core component of our characterization services. We utilize advanced mass spectrometry and other spectroscopic methods to identify and quantify PTMs.
Customer Review
-
Reliable Functional Assays
Creative Biolabs' expertise in functional assay development and execution was invaluable. Their ADCC and CDC assays provided clear, reproducible results that were essential for down-selecting our lead candidates for in vivo studies. - Dr. A***a K
-
Overcoming Aggregation Challenges
We had a particularly challenging antibody candidate prone to aggregation. Creative Biolabs' in-depth biophysical characterization, utilizing techniques like SEC-MALS and DLS, not only identified the aggregation pathways but also suggested engineering strategies. - Dr. M***n T
Related Services
To further support your drug discovery and development efforts, Creative Biolabs offers a suite of complementary services designed to seamlessly integrate with your antibody screening and characterization projects.
Antigen-specific T Cell Response Analysis
Creative Biolabs quantifies and characterizes antigen-specific T-cell responses to understand tumor-immune interactions. Their specialized team provides reliable data to advance projects quickly, determining the immune system state and capability.
Learn More →
LC-MS-based Bioanalysis Service
Liquid chromatography-mass spectrometry (LC-MS) is a foundational drug discovery tool, enabling accurate bioanalysis of small molecules, peptides, proteins, and biomarkers. It's instrumental for understanding pharmacokinetics, toxicokinetics, bioavailability, and exposure-response, accelerating drug development and ensuring safety/efficacy.
Learn More →
Contact Us
Creative Biolabs' years of expertise and proprietary technologies enable us to offer a full range of services covering all screening and characterization requirements. All of these services can be seamlessly integrated with our custom Antibody Engineering services. For any additional details or inquiries, please do not hesitate to contact us.
Reference
-
Alhazmi, Hassan A., and Mohammed Albratty. "Analytical techniques for the characterization and quantification of monoclonal antibodies." Pharmaceuticals 16.2 (2023): 291. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3390/ph16020291



 Fig.1 Four evolutionary stages of mAb.1
Fig.1 Four evolutionary stages of mAb.1
