EXLow™ Non-Detergent Assay
Endotoxin is the major contributor to the pyrogenic response caused by formulation ingredients, contaminated pharmaceutical products, and medical devices. Creative Biolabs provides EXLow™ non-detergent assay to reduce endotoxin in proteins to very low levels.
Background
Endotoxin is a complex lipopolysaccharide (LPS), derived from the outer membrane of gram-negative bacteria such as E. coli, which contributes to the organization and stability of the membrane and are released into the circulation upon disruption of the membrane by cell death. Endotoxin consists of three regions: A core polysaccharide, a long chain polysaccharide, and a nonpolar lipid called Lipid A. The core polysaccharide has an outer hexose region and an inner heptose region and the long-chain polysaccharide is a strain-specific surface antigen (O-antigen) that consists of repeating oligosaccharide subunits. The core polysaccharide and the O-antigen are both hydrophilic while lipid A is hydrophobic. Lipid A is the toxicity part of endotoxin and it triggers the production of proinflammatory cytokines and activation of the coagulation cascade which can lead to sepsis and septic shock. At some level, endotoxin is present in all untreated materials, including laboratory equipment. Thus, due to this toxicity, it is necessary to remove endotoxin.
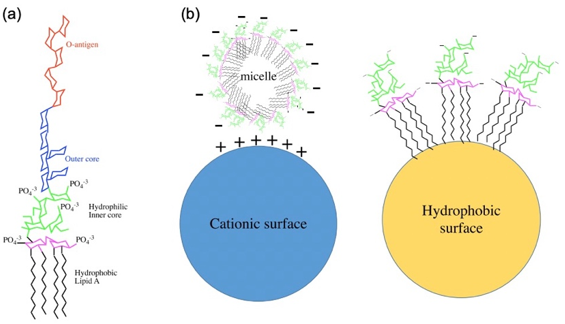 Fig.1 Overview of the endotoxin from Escherichia coli. (a) Chemical structure of endotoxin. (b) Endotoxin exhibits a net negative charge in pharmaceutical solutions. (Schneier, 2020)
Fig.1 Overview of the endotoxin from Escherichia coli. (a) Chemical structure of endotoxin. (b) Endotoxin exhibits a net negative charge in pharmaceutical solutions. (Schneier, 2020)
Endotoxin is characterized as highly stable and resistant to destruction by heat or pH. In addition, endotoxin may form stable interactions with target therapeutic compounds that further complicates separations. The detection and removal of endotoxin are crucial for the safety of patients all over the world who rely on the purity of treatments prescribed. Especially for proteins used as therapeutics, the level of endotoxin must be reduced to extremely low levels to avoid unwanted cellular and immune responses. Usually, endotoxin can be removed by affinity chromatography and/or detergent treatment. However, these approaches either may ultimately denature protein and negatively impact the desired product or do not lower endotoxin to levels acceptable for pharmaceutical discovery and development purposes. In the all above situations, the protein product must be repurified for further use. For these reason, Creative Biolabs has launched the EXLow™ non-detergent method in particular to solve the above problems.
Our EXLow™ Non-detergent Assay
-
Starting with exhaustively cleaned and validated equipment.
-
Endotoxin removal is managed at the first purification step.
-
We use detergent-free chromatography methods to lower endotoxin levels below detection of standard LAL test systems.
-
We have successfully expressed and purified multiple proteins and enzymes and reduced endotoxin to levels below 0.1 EU/mg (0.005 EU/mL).
Creative Biolabs has been committed to the research and development of multiple removal assay of endotoxin. We try our best to assure your protein will be measured by its target activity and not hampered by its endotoxin content. For more detailed information, please feel free to contact us or directly send us an inquiry.
Reference
-
Schneier, M.; et al. Current technologies to endotoxin detection and removal for biopharmaceutical purification. Biotechnology and Bioengineering. 2020.
For Research Use Only | Not For Clinical Use


 Fig.1 Overview of the endotoxin from Escherichia coli. (a) Chemical structure of endotoxin. (b) Endotoxin exhibits a net negative charge in pharmaceutical solutions. (Schneier, 2020)
Fig.1 Overview of the endotoxin from Escherichia coli. (a) Chemical structure of endotoxin. (b) Endotoxin exhibits a net negative charge in pharmaceutical solutions. (Schneier, 2020)
 Download our brochure
Download our brochure