Biologics Testing Services
Overview Featured Services Workflow Publication Why Choose Us FAQs Customer Review Related Services Contact Us
Overview
A biological assay is a procedure for determining the efficacy of a pharmaceutically active substance in a
formulated product or bulk material by observing their effects on living animals (in vivo) or
tissues (in
vitro), which produce detailed information about the biological activity of a drug substance.
Biological
assays also include the detection of biohazards, the quality assessment of mixtures, their impact on the
surrounding environment, and the assessment of the environmental impact and safety assessment of new
technologies and facilities.
With expertise in the field of oncology immunotherapy, Creative Biolabs offers the most
comprehensive
portfolio of customized biologics testing services throughout the drug development cycle. The development,
optimization, validation and performance of our biological / potency assays are carried out in accordance
with regulations. Validation strategies are based on international guidelines and recommendations
(e.g.,
ICH, US-FDA, ISO, EMEA).
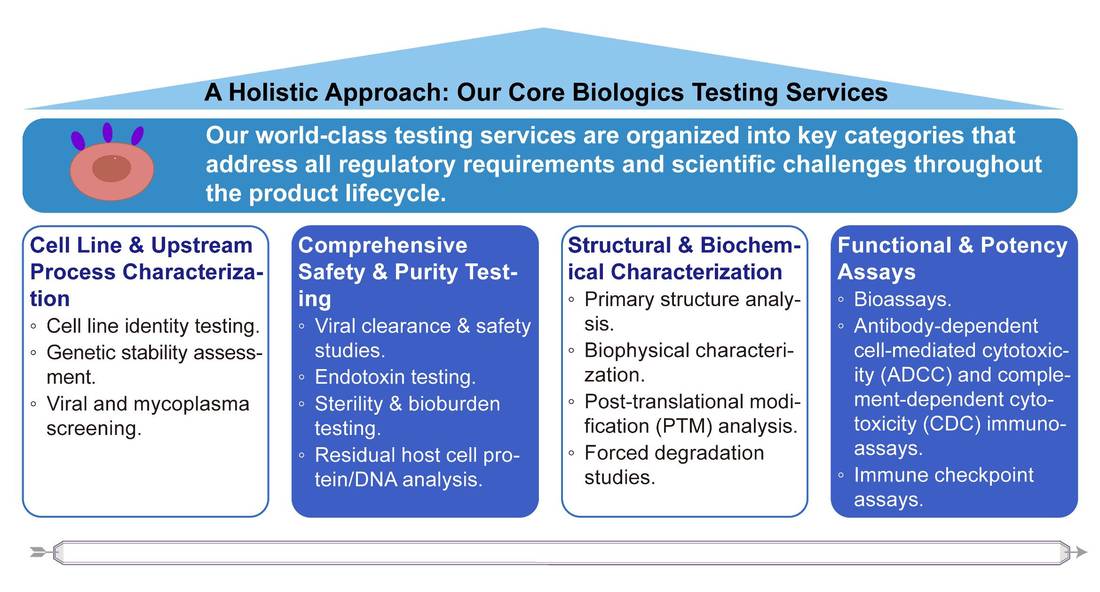
Biologics Testing Services Available
Bioassays
Bioassays determine a substance's efficacy by observing its effects on living systems. Creative Biolabs, with extensive expertise and experience, offers tailored bioassay services that include development, optimization, and validation. Our services are conducted by standard regulations and international guidelines, providing detailed information about a drug's biological activity.
Learn More →
Structure and Biochemical Characterization
Therapeutic proteins are biochemically, biophysically, and biologically characterized during clinical development to understand their structural and functional properties. Creative Biolabs provides biochemical and biophysical characterization for drug substances and products in the later stages of development, adhering to guidance. The characterization details are tailored to the clinical stage of the product.
Learn More →
Endotoxin Testing
Lipopolysaccharide exposure can cause a systemic inflammatory reaction. To ensure product safety, Creative Biolabs offers endotoxin testing to efficiently detect and remove bacterial endotoxin from injectables, drugs, and other biological and pharmaceutical products. Our extensive research and advanced technology ensure reliable and sustainable testing.
Learn More →
Our Comprehensive Biologics Testing Workflow
Biologics testing is a systematic and holistic process of analyzing a biological product to confirm its identity, purity, potency, and safety. Unlike small-molecule drugs, biologics are highly sensitive to their environment and manufacturing processes. Therefore, testing must cover every stage of development, from the initial cell line characterization to final drug product release.
Publication
This publication is a review of immune functional assays (IFAs) and their use in precision medicine. The paper highlights that an unbalanced immune response is involved in many diseases, and assessing a patient's immune profile is a challenge due to inter-individual variability. IFAs, which are assays that record a response to a given stimulation, are presented as a potential solution to this challenge.
The review discusses the use of IFA in various fields, including:
-
Diagnosing and managing infections: The publication uses tuberculosis as an example, detailing the progression from the classical tuberculin skin test (TST) to more modern interferon-gamma release assays (IGRAs).
-
Immune monitoring: The article explains how IFAs are used in diagnosing primary immune deficiencies (PIDs), managing allergies, and monitoring patients after solid organ or hematopoietic stem cell transplantation.
-
Sepsis: The review emphasizes the potential for IFAs to provide a comprehensive insight into immune functionality at the bedside, which is crucial for managing the dynamic and complex immune responses in sepsis.
The authors conclude that the design of new tools that provide a comprehensive and timely insight into immune functionality represents a significant step toward patient immunoprofiling.
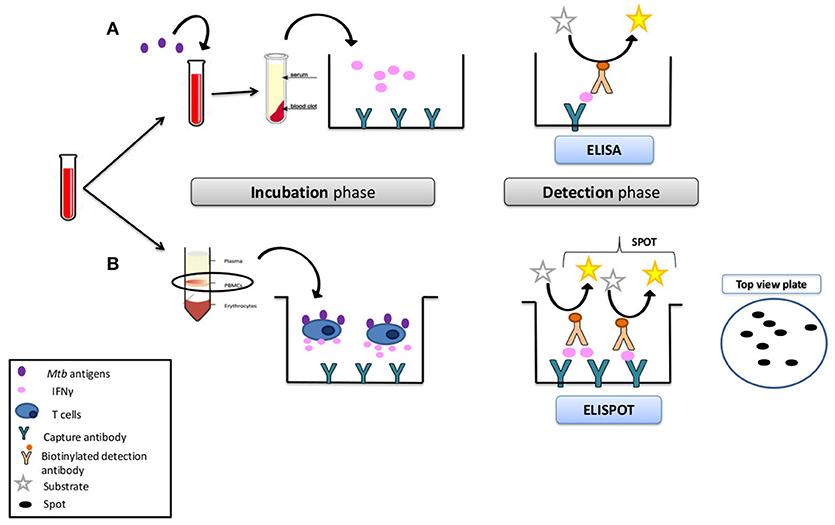 Fig.1 In vitro assays utilizing interferon-gamma.1
Fig.1 In vitro assays utilizing interferon-gamma.1
Why Choose Us?
Our deep scientific expertise and unwavering commitment to quality are what set Creative Biolabs apart. We offer a holistic, integrated approach that addresses the unique challenges of biologics, from complex molecular characterization to non-clinical safety testing. We understand that non-clinical safety testing for biologics presents distinct challenges, and our expertise includes the careful selection of appropriate animal models, such as minipigs, as an alternative to non-human primates where relevant. This strategic approach helps to refine the use of animal models and improve the predictability of safety profiles. Furthermore, our ability to conduct in-depth distribution studies provides invaluable insights into unexpected clearance mechanisms, a critical factor for complex molecules like bispecific antibodies.
Creative Biolabs' team of scientists is dedicated to ensuring the most appropriate method, based on the project, to address the needs of clients. For more information on biologics testing, please contact us immediately.
FAQs
Q1: What types of biologics do you test?
A1: We offer comprehensive testing for a wide range of biologics, including monoclonal antibodies, antibody-drug conjugates (ADCs), therapeutic proteins, and cell therapies. Our flexible platform allows us to customize our services for almost any biologic modality.
Q2: What if my biologic has an unexpected off-target effect during non-clinical testing?
A2: Our team of experts is highly experienced in interpreting complex safety data. We will work with you to understand the underlying mechanisms of any unexpected effects and propose solutions, such as exploring alternative animal models like minipigs or adjusting your development strategy.
Q3: How do your functional bioassays differ from a simple binding assay?
A3: While binding assays confirm that your biologic can bind to its target, a functional bioassay provides a true measure of its biological activity and potency. This data is often a critical requirement for regulatory approval and is a key indicator of therapeutic efficacy.
Customer Review
-
Accurate Potency Data
The immune functional assays from Creative Biolabs were essential for our immuno-oncology program. The ADCC and CDC data were highly reproducible and provided the critical potency information we needed to select our lead candidate. - A***n L
-
Key Insights on Stability
The biophysical characterization data we received from Creative Biolabs on our therapeutic protein was invaluable. Their analysis of aggregation and thermal stability helped us optimize our formulation, greatly improving the product's shelf life. - L***n S
Related Services
To further support your biopharmaceutical project, Creative Biolabs offers a range of complementary services:
T Cell-based Bioassay
Creative Biolabs provides cancer epitope analysis assays, including a T cell-based bioassay service. This service identifies potential T cell epitopes by assessing cytokine release levels elicited by stimulation. This method is used to evaluate immunotoxicity and characterize cell responses.
Learn More →
System Immunity Measurement
The gut microbiota and immune system influence each other. Creative Biolabs provides a wide range of bioassay and multi-omics services for measuring systemic immunity in gut microbiota-associated neuro diseases.
Learn More →
Contact Us
Creative Biolabs is your trusted partner for navigating the complexities of biologics development. Our comprehensive testing services are designed to ensure your product's safety, purity, and potency, giving you the confidence to accelerate your path to market.
Contact our team today to discuss your project, and let's work together to achieve your next breakthrough.
Reference
-
Albert-Vega, Chloé, et al. "Immune functional assays, from custom to standardized tests for precision medicine." Frontiers in immunology 9 (2018): 2367. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3389/fimmu.2018.02367



 Fig.1 In vitro assays utilizing interferon-gamma.1
Fig.1 In vitro assays utilizing interferon-gamma.1
