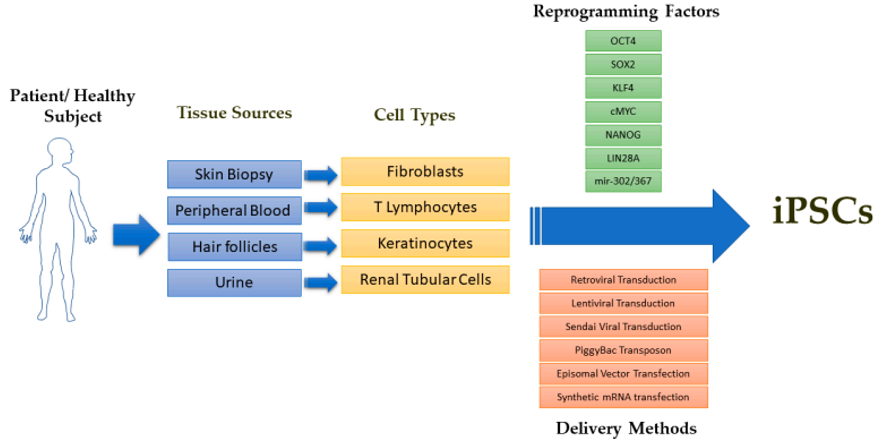
 Fig. 1 Schematic overview of iPSC derivation from a patient or healthy subject.1
Fig. 1 Schematic overview of iPSC derivation from a patient or healthy subject.1
iPSCs are generated by reprogramming somatic cells and possess unique properties of self-renewal and differentiation to many types of cell lineage. They were firstly generated from mouse fibroblasts by the introduction of four transcription factors (Oct4, Sox-2, c-Myc, and Klf-4) through genetic reprogramming. With the guide of different protocols, iPSCs can differentiate into any type of cells theoretically, including neural cells, cardiomyocytes, chondrocytes, retinal pigment epithelial cells, pancreatic islet cells, and hepatocytes.
iPSCs have become attractive candidates for cell therapy-based regenerative medicine. Moreover, patient-derived iPSCs can be applied in multiple critical in vitro studies, such as in vitro disease modeling, toxicity screenings, drug development, drug delivery, etc.
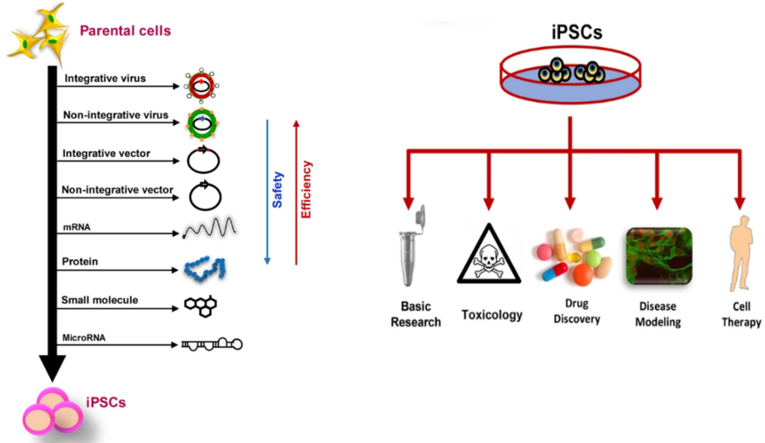 Fig.2 Generation and applications of iPSCs.2
Fig.2 Generation and applications of iPSCs.2
| Applications | Descriptions | Advantages | ResearchAreas |
|---|---|---|---|
| iPSC-derived In vitro Model | iPSCs are valuable in studying the molecular mechanisms of many diseases. They have been widely used for disease modeling and gene therapy for various diseases. |
|
|
| iPSC for Cell Therapy | iPSC-based cell therapies have proven to be very powerful and important in biomedical research and personalized regenerative medicine. The first step in iPSC therapy is the differentiation of the iPSC into the desired target cell type, and the resulting specialized tissue-specific cells are then transplanted into the patient as a cell suspension or more complex tissue structure. |
|
|
| iPSC as Drug Delivery Vehicles | iPSCs as drug delivery vehicles represent an innovative approach to therapeutic delivery, combining the benefits of cell therapy with targeted drug delivery. |
|
|
| iPSC for 3D Bio-printing | 3D bioprinting of iPSC or iPSC-derived cells to create multi-scale tissue structures has evolved as a tool for creating regenerative medicine and patient-specific therapies. The technology is able to generalize the microstructure of specific tissues and facilitate tissue engineering purposes, i.e., replacement or regeneration of damaged and diseased tissues. |
|
|
| Organoids | Organoids are 3D cell cultures grown in vitro that generalize key features of organs in vivo. Because organoids have the genetic and physiological similarities required for human disease modeling, they can be used for disease modeling and drug screening applications to assess efficacy and safety. |
|
A range of organoids have been developed, including
|
| iPSC Derivatives | iPSC and iPSC derivatives (growth factors, cytokines, differentiated histiocytes/progenitor cells, etc.) can be used as a source of autologous bioactive agents that can be used for future clinical translation to treat various diseases. |
|
|
A full range of one-stop services for iPSC research
iPSC can differentiate into a variety of cell types, including neurons, cardiomyocytes, and hepatocytes, which closely resemble natural cells. This enables researchers to create in vitro models that more accurately replicate the biological microenvironment of human tissues, identifying promising therapeutic candidates through reliable and dependable high-throughput screening.
Provide a relevant platform for neurodegenerative diseases such as Alzheimer's or Parkinson's, allowing precise assessment of neuroprotective or neurotoxic effects.
Provide physiologically relevant models for assessing ion channel modulators and cardiovascular safety.
One of the biggest obstacles to successful drug development is the accurate prediction of toxicity. iPSC-derived hepatocytes and cardiomyocytes are now addressing this challenge.

iPSC-derived hepatocytes exhibit key metabolic functions, including cytochrome P450 activity, enabling researchers to study drug-induced liver injury (DILI).
iPSC-derived cardiomyocytes exhibit spontaneous beating and electrophysiological features such as action potentials and calcium signaling.
The unique ability of iPSC to recapitulate human disease phenotypes stems from its pluripotency and ability to differentiate into virtually any cell type.
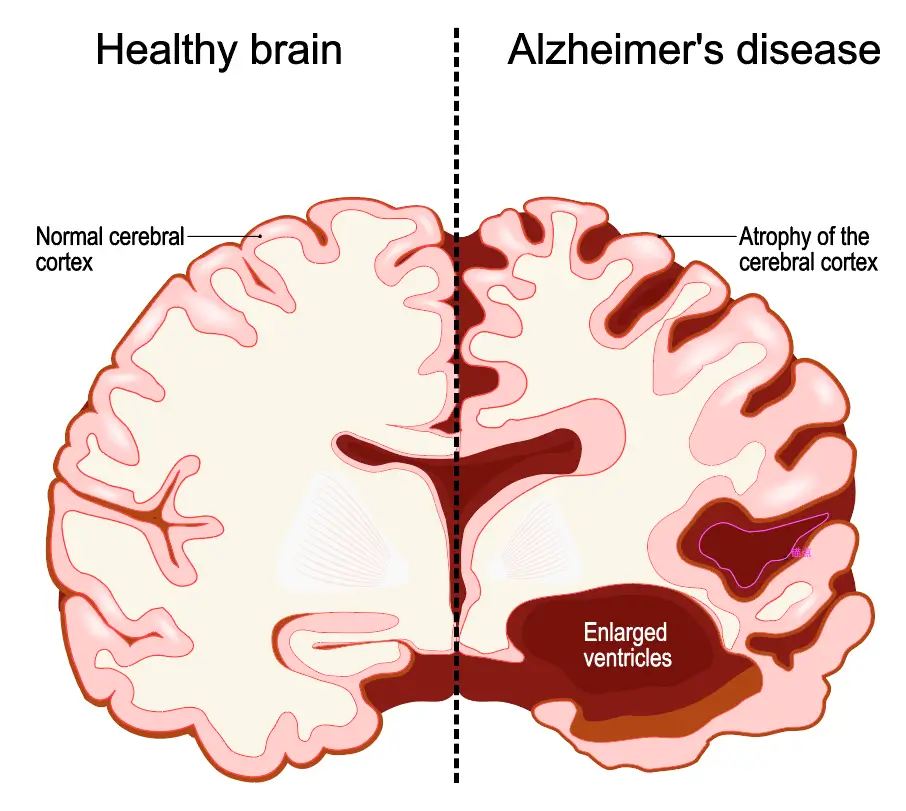
iPSC-derived models provide a platform for screening drugs that target the amyloid pathway or synaptic elasticity.
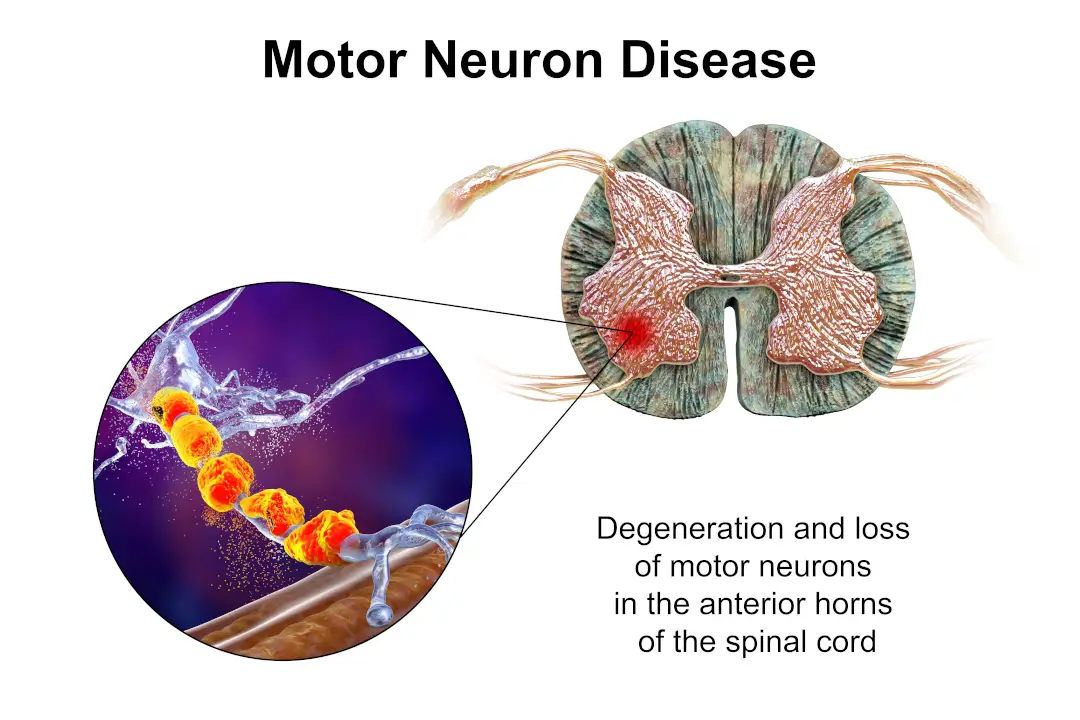
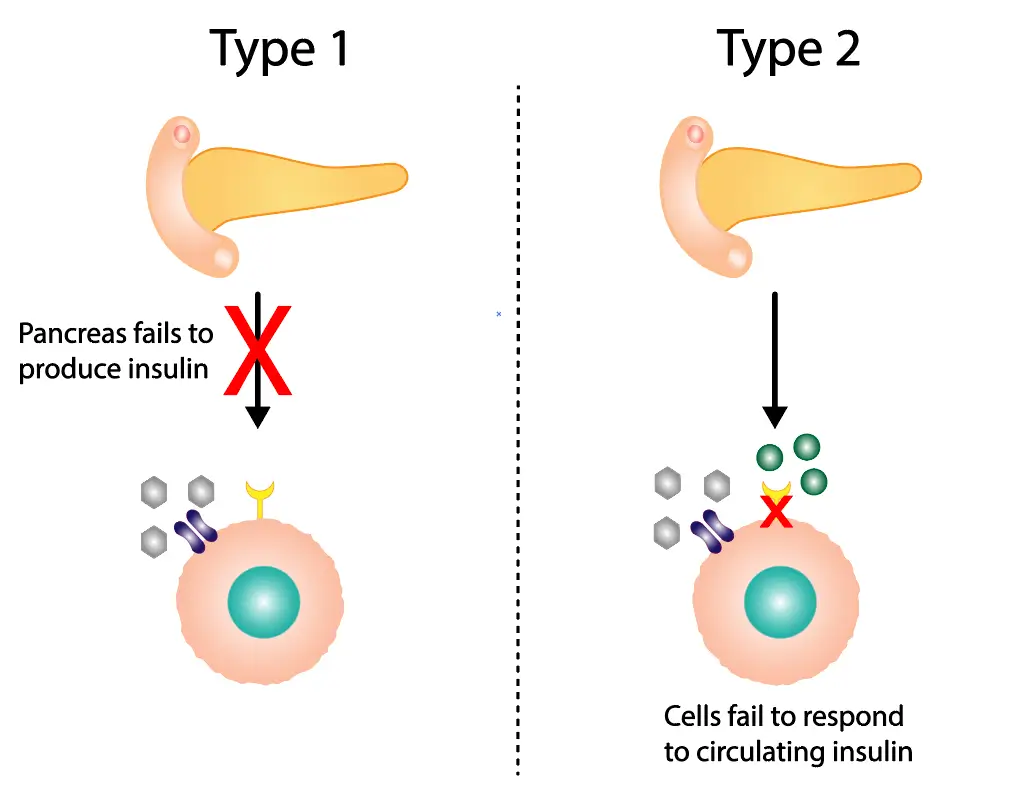
iPSCs have pluripotency similar to ESCs and are capable of generating a wide range of cell types, such as neurons, cardiomyocytes, and hepatocytes. This property lays the foundation for their use in modeling human development and disease.
Because iPSCs can be derived from an individual's somatic cells, they eliminate the risk of immune rejection and open up avenues for personalized regenerative therapies.
Unlike ESC, iPSC bypasses the controversial use of embryos and meets ethical and regulatory guidelines around the world.
iPSC has enabled the development of organoid structures that recapitulate the structure and function of organs such as the liver, brain,and kidney.
Tissue engineering combines iPSC with biomaterials to create functional tissues. Advances in bioprinting technology have enabled the creation of vascularized tissues that closely resemble natural tissues.
iPSC-derived cardiomyocytes can be integrated into damaged heart tissue to restore contractile function and improve overall cardiac function.
The generation of functional neurons and glial cells from iPSC has facilitated the development of strategies to repair spinal cord injuries and neurodegenerative diseases.
iPSC-derived cells can be inoculated into hydrogels with tunable properties to provide a favorable microenvironment for cell growth and differentiation.
Decellularized tissues implanted with iPSC-derived cells create biocompatible scaffolds that mimic the natural extracellular matrix, allowing the construction of bioengineered organs such as lungs and kidneys.
Recent developments in reprogramming methods have improved the efficiency and safety of iPSC generation, including small molecule compound regulation, mRNA technology, and CRISPR activation.
Customized protocols that closely mimicin vivo conditions are being developed to address heterogeneity. Some of the solutions used include 3D culture systems, single cell analysis, and artificial intelligence.
Maintaining genetic integrity is critical for reliable use of iPSCs, by limiting the time of iPSC culture, using genome monitoring tools, non-integrated reprogramming techniques, etc.
Integration of histology data with iPSC research facilitates better understanding and application, such as combining genomics, transcriptomics, and proteomics data, utilizing cloud-based platforms, etc.
References