All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs has a professional scientist team with wide background of T cell receptor (TCR) engineering and analysis experience. We are committed to offering TCR clonality assessment service in a most time and cost effective manner to meet the specific requirements of customers.
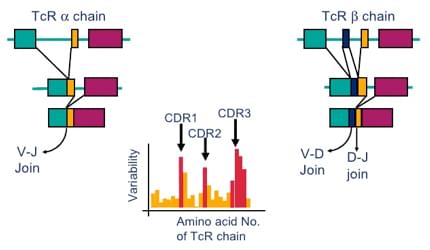
The T-cell receptor is a complex of integral membrane proteins that participates in the activation of T-cells in response to an antigen, which plays a vital role in our body defence, and any dysregulation in the process of TCR development can cause certain kinds of diseases. TCR clonality analysis is an efficient method for the principal diagnosis of lymphoid malignancies and other immune system diseases. The most common aim of our TCR clonality analysis service is to determine if there is significant monoclonal expansion of T-cells and to correlate this information with clinical, histological and immunohistochemical data to help customers distinguish between reactive and malignant lymphoproliferation. Based on highly polymorphic gene markers, clonality analysis has become a useful and common tool to analyze the rearrangement of TCR genes or other genes. Creative Biolabs now provides an elaborately analytical platform for customers to perform the assessment of TCR clonality of interest based on various mature analysis approaches. At Creative Bilobs, the classical diagnostic method for TCR clonality assessment, southern blot (SB) analysis is always available since it is highly reliable and in principle can detect every clonal TCR gene rearrangement. Furthermore, TCR clonality analysis based on polymerase chain reaction (PCR) is strongly recommended and it is the most widely used method for detection of TCR rearrangements in the clinical laboratory , an obvious advantage of which is that it allows quicker performing and using poor quality DNA . In addition, Creative Biolabs processes years of experience in the field of next-generation sequencing, we can also perform next-generation sequencing (NGS)-based strategy to determine T-cell clonality, which shows superior sensitivity compared with traditional approach. Meanwhile, we also provide TCR clonality analysis for patients suspected with lymphoproliferative diseases or lymphocythaemia in animal models for your project.
With access not only to the comprehensive analytical technologies, but also to experienced staff with expertise in designing and conducting TCR- related experiments and analyzing data, our TCR clonality analysis platform creates virtually convenience for detecting TCR clonality and is unprecedented in the ease of promoting your clinical and scientific research.
To discuss your demands or to request a proposal, please contact us by .
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION