All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
The use of CAR-T cells has been demonstrated to be a promising treatment against hematological malignancies with successes in the autologous setting. Despite the encouraging results achieved in the clinical setting, tumorigenicity and off-target toxicity are still the main concerns of its clinical application. The non-specific activation of patient T cells not only induces cytotoxic activity against tumors, but also induces harmful toxicity against normal tissues, leading to immune-related adverse events. Therefore, to perform T cell therapy safely and effectively, immunotherapy using T cells that can accurately separate tumor cells from normal cells is required.
To clarify the characteristics of antitumor CAR more precisely for expansion of treatment modalities, Creative Biolabs provides cross tissue reactivity study services to help customers investigate the potential target-specific reactivity of CAR-redirected T cells in vitro and in vivo.
For the rest of the plan, this stage is the most critical and important. At Creative Biolabs, we are most concerned about the development of the initial protocol to ensure the smooth progress of the subsequent GLP phase. If feasible, the method will be developed on an automated platform, if not, the method will be developed manually. Method development will be completely driven by science and data.
Our assay development complies with the most recent guidelines on analytical validation. Our fit-for-purpose validation is very strict, allowing us to inform you promptly on the outcome of the method validation progress in time. You will immediately be notified of the most critical verification parameters to quickly help you with the next steps.
To improve the staining conditions (matrix effect) of various tissues, especially the preliminary assessment of background staining in different tissue types, and the preliminary assessment of possible targets or target staining, a non-GLP screening phase may be conducted. This may also be useful for the preparation of the final GLP study.
The common format of this method is twelve tissues from two different donors. TMA (tissue micro array) format can also be used to screen all tissues required by FDA and EMA, especially for first-class biopharmaceuticals. Here, before conducting in vivo safety (toxicology) studies, model animal species can be screened after verifying cross-species reactivity.
The list of 33 tissues recommended by the FDA and screened as part of this study:
In the third stage, in accordance with the requirements of the FDA and EMA, the main GLP study was conducted on 38 panels of human tissues/organs from at least three unrelated, unaffected donors.
If automatic methods are used, the turnaround time for these studies (including the reporting phase) is 2 months, and if manual methods are used, it is 3 months. The data is entered into an Excel file, printed, dated, and signed. Our quality check will ensure the integrity of the data, and our report will contain visibly stained images.
There are many applications for TCR analysis. They include:
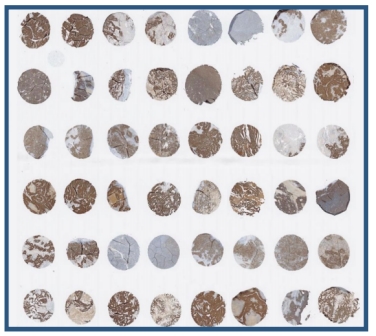
Our cross-reactivity assays follow the FDA and EMEA guidelines :
For more detailed information about our technology platform for TCR assay, please visit:
Surfarray™ Cell Microarray Technology
Please feel free to contact us or directly sent us an inquiry.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION