All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 Cancer vaccines are complex and costly products, with a high risk in long-term research, development, and manufacture. Thus, it is necessary to maximize the potential of rapid formulation development. To achieve this goal, the historical methods for formulation development needs to be updated in a rational and systematic way allowing for better cancer vaccine formulations.
Cancer vaccines are complex and costly products, with a high risk in long-term research, development, and manufacture. Thus, it is necessary to maximize the potential of rapid formulation development. To achieve this goal, the historical methods for formulation development needs to be updated in a rational and systematic way allowing for better cancer vaccine formulations.
In Creative Biolabs, we offer a diversity of approaches involving biophysical characterization of antigens, evaluation of stabilizers, investigation of interactions between antigen and adjuvants, assessment of product contact materials, and stability monitoring both in real-time and accelerated conditions. With the information gained from these studies, there is a great increase in the probability of rapidly developing a safe, effective, and stable cancer vaccine formulation.
There are two main goals of formulation development. One is to improve the immunogenicity of the vaccine and the other is to improve the stability of vaccine components. At Creative Biolabs, we have designed comprehensive services for cancer vaccine formulation optimization, and each of which takes place in multiple phases of development and is complementary to each other. Besides, our teams have rich experience and dedicated equipment to predict and address potential issues in formulation development processes for the supply of high-quality cancer vaccines.
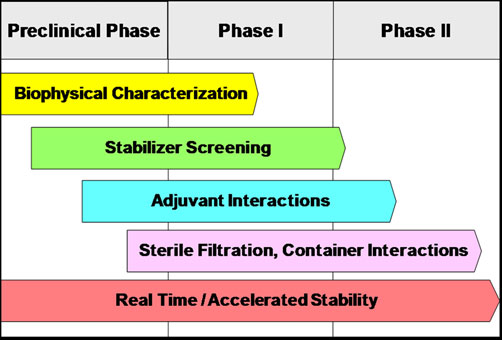
Fig.2 Components of a rational and systematic approach to the development of vaccine formulations. (Morefield, 2011)
As an expert in the vaccine industry, Creative Biolabs can provide access to adjuvants, bespoke adjuvant R&D activities, adjuvant quality control technology, as well as formulation expertise, training, access to GMP adjuvants, so that customers gain optimal benefit from the use of cancer vaccine formulation technology.
For more details regarding the formulation development of cancer vaccines, please don't hesitate to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION