All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs is a forward-looking CRO company as well as a leading custom service provider in the field of CAR-T therapy. We are using powerful algorithms to search and analyze quadrillion CAR components to design VZV delivery systems with precise targeting capabilities. We also use high-throughput genomics and artificial intelligence to identify CAR and host-spot antibodies for optimal pharmacology.
Varicella-zoster virus (VZV) is one of eight herpes viruses known to cause infection in humans. VZV infection clinically causes two different forms of the disease: chickenpox and shingles. VZV belongs to the alpha-herpesvirus family and is caused by the reactivation of viruses lurking in the dorsal sensory ganglia. Detection of VZV reactivation or impaired immune control by VZV can generally be used to understand the risk or progression of severe viral infections. Recently, due to virus-induced innate and acquired immunity, many researchers have tried to combine virus-specific T cells (VST) with cancer-specific chimeric antigen receptor (CAR), aiming to use the VZV immunogenicity to treat solid tumors.
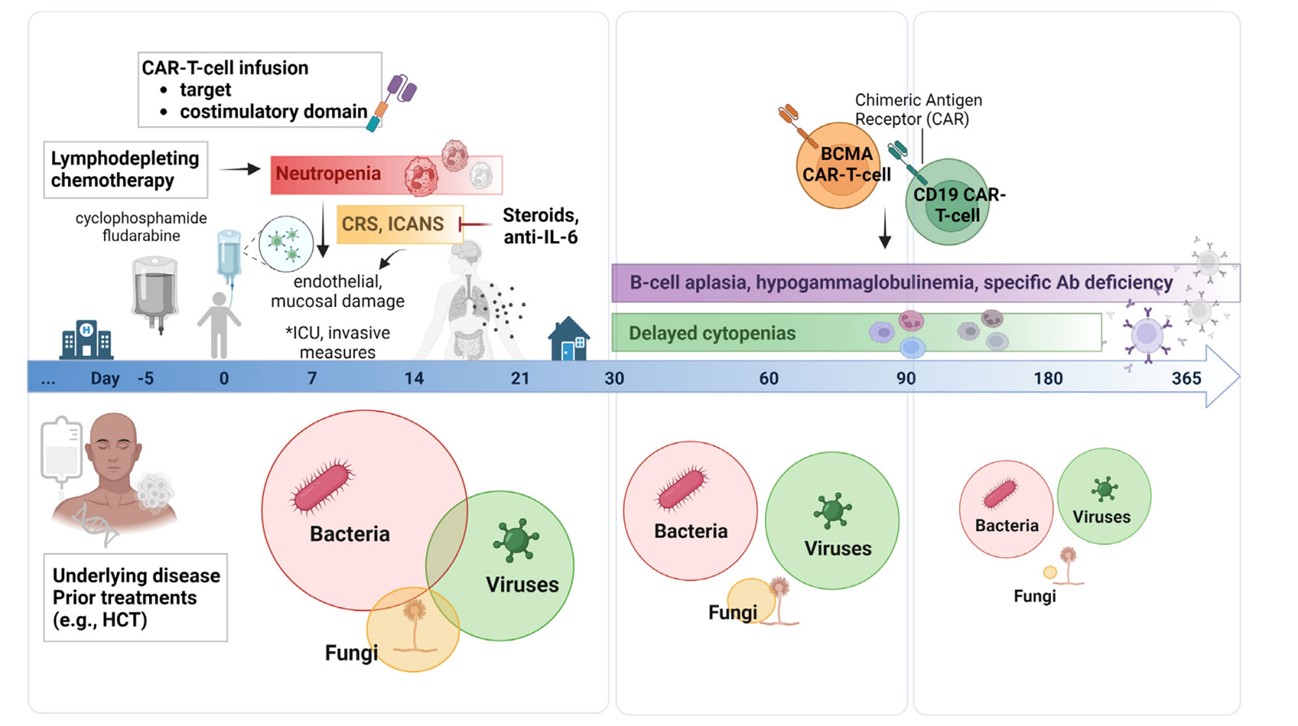 Fig.1 Infection Risk And Epidemiology During Different Time Intervals After CAR-T Cell Therapy.1
Fig.1 Infection Risk And Epidemiology During Different Time Intervals After CAR-T Cell Therapy.1
Creative Biolabs is a CRO company with operations in the United States focused on innovative CAR-T cell therapies for the treatment of hematological malignancies and solid tumors. Up to now, we have developed a range of virus-based vaccine-boosting CAR-T platforms, including target discovery, custom CAR-T cell development, and CAR-T cell characterization. We overcome many of the challenges of traditional CAR-T cell therapy in the treatment of solid tumors. In our labs, by stimulating peripheral blood mononuclear cells from healthy donors and cancer patients with overlapping peptide libraries containing selected VZV antigens, our staff have generated GMP-compliant GD2 CAR-modified VZVST and then tested its ability to recognize and kill both GD2- and VZV antigens. After VZV vaccination, we first validated the T cell specificity of the VZV antigen. Cytokines secreted by VZVST can respond to VZV antigen, kill VZV-infected target cells, and inhibit the spread of infected viruses. Furthermore, our data have indicated that GD2 CAR-modified VZVST showed a stronger and faster tumor-killing effect.
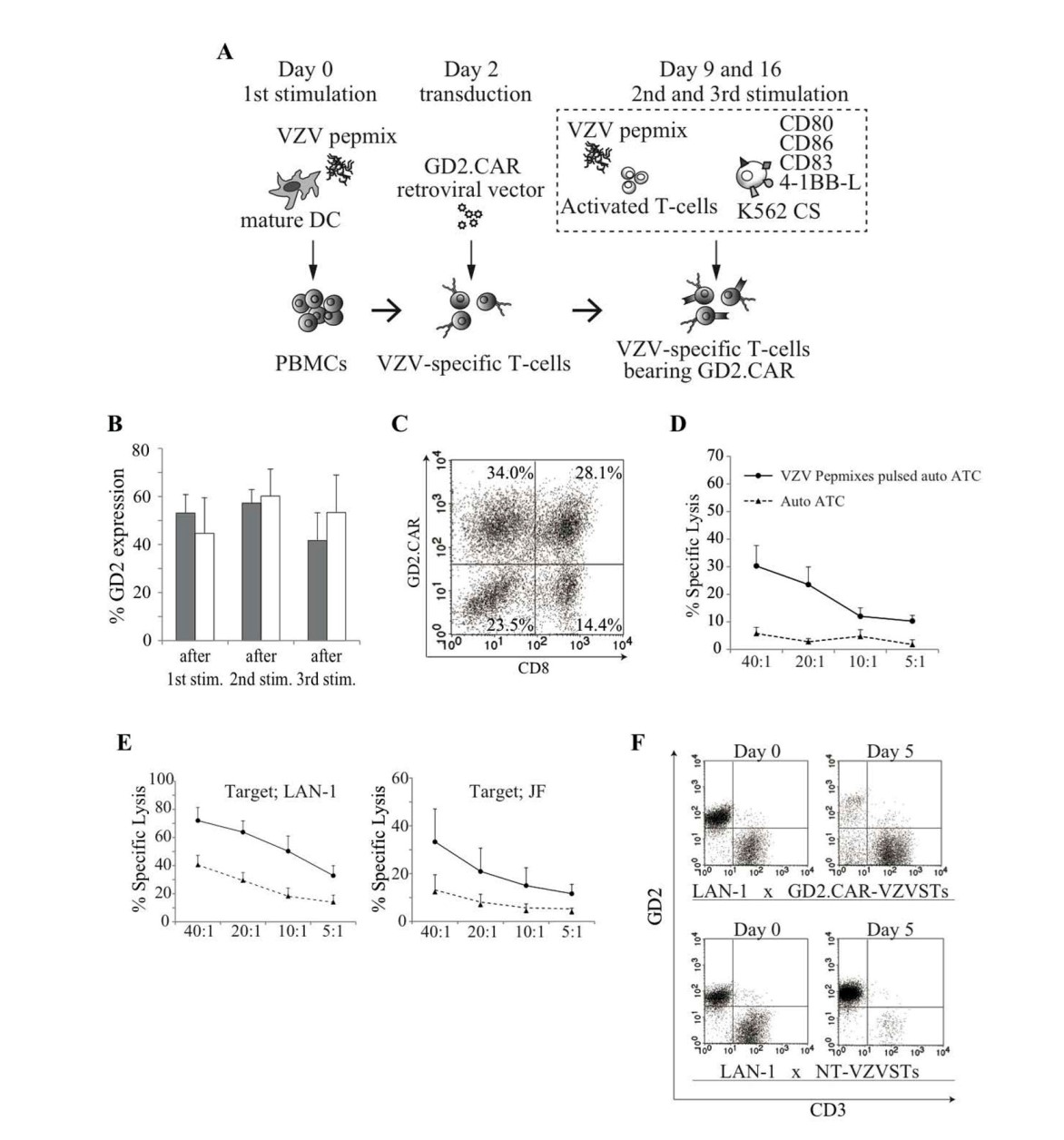 Fig. 2 CARs Can Be Expressed In VZVSTs And Have Functional Specificity For Both VZV And GD2.2
Fig. 2 CARs Can Be Expressed In VZVSTs And Have Functional Specificity For Both VZV And GD2.2
Creative Biolabs is developing its proprietary CAR-VZVSTs technology for the treatment of solid tumors. Our approach is designed to VZVSTs transduced with a retroviral vector encoding scFv CAR constructs.
Using VSTs as a novel platform for CAR expression can overcome a few limitations of uniserval CAR-T cell therapy for solid tumors by stimulating natural TCRs, and promoting expansion and persistence by stimulating the native TCR using highly immunogenic viral vaccines or oncolytic viruses. Also, the interference of CAR-VZVSTs on TCR function does not depend on the ligation of antigen to CAR.
Vaccination by TCR can reactivate the dysfunctional CAR-T cells caused by the tumor microenvironment. It is of great significance for controlling cancer progression. We have established strong in-house vertically integrated production capabilities, including the production of CAR plasmids, CAR disease vectors, and custom CART cells.
Creative Biolabs is now developing its lead CAR-VZVSTs compound to enable pre-clinical evaluation. Our technology could be used in conjunction with traditional CAR-T or TCR cell therapies to overcome obstacles in the treatment of solid tumors. Our vision is to address the anti-tumor bottleneck by specifically delivering a CAR to the virus-specific vaccine. If you are seeking a reliable partner to advance your CAR development, please contact us immediately for outstanding services.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION