All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs is a Contract Research Organization (CRO) founded and run by a team of high skilled scientists and experts in the field of cell therapy research and development. Our mission is to offer top-notch products and services to clients worldwide, spanning both academic and industrial sectors. We are committed to facilitating breakthrough scientific discoveries and driving progress in preclinical and clinical research.
CD123 is an alpha subunit receptor that belongs to the interleukin 3 (IL-3) family of cytokines. It is predominantly expressed in hematopoietic tissues and leukemic cells, as well as fibroblasts, dendritic cells, and macrophages. CD123 plays a crucial role in regulating the proliferation and differentiation of stem cells and leukocytes during hematopoiesis. Its structure comprises an external N-terminal domain, a membrane domain, and an internal C-terminal domain. CD123 expression levels are often associated with malignancy, prognosis, and the risk of metastasis. In acute myeloid leukemia (AML), high levels of CD123 expression are linked with poor prognosis, relapse, and metastasis, making it a promising target for AML treatment. CD123 is also of interest in other malignancies, such as myeloproliferative neoplasms (MPN), multiple myeloma (MM), and chronic lymphocytic leukemia (CLL).
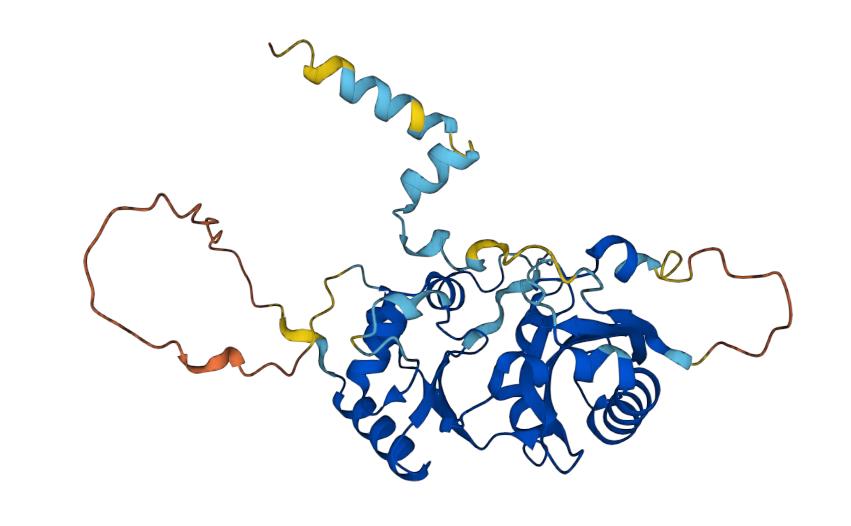 Fig.1 Structure of CD123
Fig.1 Structure of CD123
Both CD123 and IL-3 are molecules associated with the immune system. CD123 acts as a membrane receptor that plays a significant role in dendritic cells, macrophages, and mast cells. IL-3 is a cytokine that, by binding to CD123, forms a trimeric complex with other subunits IL-3Rβ and γc, thereby activating downstream signaling pathways. The IL-3/CD123 signaling pathway is involving in numerous biological responses, including cell proliferation, survival, and differentiation. In dendritic cells, activation of the IL-3/CD123 signaling pathway promotes their maturation and antigen-presenting functions, facilitating the immune response of T cells. In mast cells and macrophages, activation of the IL-3/CD123 signaling pathway promotes cell proliferation and survival and is involved in physiological and pathological processes such as allergic and inflammatory responses. In addition to malignancies such as leukemia and lymphoma, the IL-3/CD123 signaling pathway plays an important role in autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), where CD123 expression is significantly increased in patients. Blocking CD123 can suppress the autoimmune response , reducing the clinical symptoms of diseases such as SLE and RA. CD123 is a promising therapeutic target in malignant tissues, playing a critical role in various cancers.
CD123 is currently being widely studied and used in chimeric antigen receptor (CAR)-T cell therapy for hematologic tumors, such as AML and chronic myeloid leukemia/myelodysplastic syndrome (CMML/MDS). Early findings suggest that CD123 CAR-T cells significantly inhibit cancer cell proliferation and have long-term viability. CD123 is a monoclonal antibody that is highly expressed in leukemia, myelodysplastic syndromes, and other hematologic malignancies, making it a potential therapeutic target. CAR T-cell therapy is a novel immunotherapeutic approach that uses modified T cells to recognize and kill cancer cells. Preclinical studies of CD123 CAR-T cell therapy have shown that this therapy induces the removal of CD123-positive leukemia cells and has a significant therapeutic effect on certain diseases. For example, an early study demonstrated that CD123 CAR-T cells effectively removed AML cells and enhanced survival in a mouse model. Another study found that CD123 expression levels were high in blood and bone marrow samples from human pre-B lymphoblastic leukemia (pre-B ALL) patients, and that CD123 CAR-T cell therapy was effective in removing leukemia cells and reducing the risk of metastasis and relapse. Based on preclinical studies, CD123 CAR T-cell therapy has entered preliminary clinical trials. Additionally, several ongoing studies are attempting to explore the combination of other types of CAR T-cell therapeutic targets with CD123 to improve treatment efficacy.
Table 1. Ongoing CD123-Targeted CAR Cell Therapy Clinical Trials
| NCT Number | Title | Status | Conditions | Sponsor/Collaborators | Phases |
| NCT05574608 | Allogenic CD123-CAR-NK Cells in the Treatment of Refractory/Relapsed Acute Myeloid Leukemia | Recruiting | Acute Myeloid Leukemia Refractory|Acute Myeloid Leukemia Recurrent | Affiliated Hospital to Academy of Military Medical Sciences|Beijing JD Biotech Co. LTD. | Early Phase 1 |
| NCT04272125 | Safety and Efficacy of CD123-Targeted CAR-T Therapy for Relapsed/Refractory Acute Myeloid Leukemia | Recruiting | Leukemia|Leukemia, Myeloid|Leukemia, Myeloid, Acute | Chongqing Precision Biotech Co., Ltd | Phase 1|Phase 2 |
| NCT04318678 | CD123-Directed Autologous T-Cell Therapy for Acute Myelogenous Leukemia (CATCHAML) | Recruiting | AML|B-ALL|T-ALL|BPDCN|MDS | St. Jude Children's Research Hospital | Phase 1 |
| NCT04109482 | Trial to Evaluate the Safety and Efficacy of MB-102 in Patients With BPDCN. | Recruiting | Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) | Mustang Bio | Phase 1|Phase 2 |
| NCT02159495 | Genetically Modified T-cell Immunotherapy in Treating Patients With Relapsed/Refractory Acute Myeloid Leukemia and Persistent/Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm | Active, not recruiting | Adult Acute Myeloid Leukemia in Remission|Acute Biphenotypic Leukemia|Early Relapse of Acute Myeloid Leukemia|Late Relapse of Acute Myeloid Leukemia|Recurrent Adult Acute Myeloid Leukemia|Secondary Acute Myeloid Leukemia|Blastic Plasmacytoid Dendritic Cell Neoplasm|Acute Myeloid Leukemia|Adult Acute Lymphoblastic Leukemia|Interleukin-3 Receptor Subunit Alpha Positive|Minimal Residual Disease|Refractory Acute Myeloid Leukemia|Untreated Adult Acute Myeloid Leukemia | City of Hope Medical Center|National Cancer Institute (NCI)|Mustang Bio, Inc. | Phase 1 |
| NCT05528887 | Study of CAR-T Cell Therapy in the Treatment of Relapsed/Refractory Hematological Malignancies | Recruiting | Relapsed/Refractory Hematological Malignancies|Lymphoma|Myeloma|Leukemia | The Affiliated People's Hospital of Ningbo University|UTC Therapeutics Inc. | Phase 1 |
| NCT04599543 | IL3 CAR-T Cell Therapy for Patients With CD123 Positive Relapsed and/or Refractory Acute Myeloid Leukemia | Not yet recruiting | Acute Myeloid Leukemia | Zhejiang University|Yake Biotechnology Ltd. | Early Phase 1 |
| NCT04010877 | Multiple CAR-T Cell Therapy Targeting AML | Recruiting | Acute Myeloid Leukemia | Shenzhen Geno-Immune Medical Institute | Phase 1|Phase 2 |
| NCT04430530 | 4SCAR-T Therapy Post CD19-targeted Immunotherapy | Recruiting | CD19 Negative B-cell Malignancies | Shenzhen Geno-Immune Medical Institute|ShiJiaZhuang Zhongxi Children Hospital|Shenzhen Children's Hospital|The Seventh Affiliated Hospital of Sun Yat-sen University | Phase 1|Phase 2 |
| NCT04016129 | CAR-T Immunotherapy Targeting CD19- ALL | Recruiting | B-cell Leukemia | Shenzhen Geno-Immune Medical Institute | Phase 1|Phase 2 |
| NCT04230265 | Dose-escalating Trial With UniCAR02-T Cells and CD123 Target Module (TM123) in Patients With Hematologic and Lymphatic Malignancies | Recruiting | Acute Myeloid Leukemia (AML) | AvenCell Europe GmbH|PHARMALOG Institut für klinische Forschung GmbH | Phase 1 |
| NCT03190278 | Study Evaluating Safety and Efficacy of UCART123 in Patients With Relapsed/ Refractory Acute Myeloid Leukemia | Recruiting | Relapsed/Refractory Acute Myeloid Leukemia | Cellectis S.A. | Phase 1 |
| NCT05457010 | Phase I Study of Cell Therapies for the Treatment of Patients With Relapsed or Refractory AML or High-risk MDS | Recruiting | Acute Myeloid Leukemia|Myelodysplastic Syndromes | Arcellx, Inc. | Phase 1 |
| NCT03098355 | Interleukin-2 Following 4SCAR19/22 T Cells Targeting Refractory and/or Recurrent B Cell Malignancies | Suspended | B-Cell Leukemia|B-Cell Lymphoma | Zhujiang Hospital|Shenzhen Geno-Immune Medical Institute | Phase 1|Phase 2 |
| NCT04678336 | CD123 Redirected T Cells for AML in Pediatric Subjects | Active, not recruiting | Acute Myeloid Leukemia, in Relapse|Acute Myeloid Leukemia, Pediatric|Acute Myeloid Leukemia, Refractory | University of Pennsylvania | Phase 1 |
| NCT03766126 | Lentivirally Redirected CD123 Autologous T Cells in AML | Active, not recruiting | Acute Myeloid Leukemia, in Relapse|Acute Myeloid Leukemia, Adult|Acute Myeloid Leukemia, Refractory | University of Pennsylvania | Phase 1 |
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION