All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
With the remarkable success of CAR-T cells for treating hematological malignancies, cell‑based adoptive immunotherapy is considered a promising treatment for various cancer types. Adoptive transfer of NK cells is a rapidly growing interest in developing CAR-engineered NK (CAR-NK) cells for adoptive immunotherapy. However, due to the lack of a unified standard protocols for producing a large number of clinical-grade effector cells, it still remains challenging to transfer the bench-top expansion protocols to clinically applicable methods for NK cell-based therapy.
At Creative Biolabs, we validated NK expansion protocols based on cytokine cocktails, which enables easily NK expansion in vitro from peripheral blood mononuclear cells (PBMC). The optimized protocols allow for large-scale NK cell ex vivo expansion for adoptive immunotherapy as a clinical application. These cytokine-induced NK cells exhibit increased antitumor effects and proliferation and are ready for preclinical and clinical applications.
Studies have shown that a variety of cytokines can regulate the development, proliferation, and activation of NK cells. Previous studies conducted with cytokine-stimulated NK cells or PBMC have shown the safety of this method and demonstrated some clinical responses after adoptive NK cell transfer. In addition, groundbreaking studies have shown that IL-2-induced NK cell activation can induce remission in AML patients without causing graft-versus-host disease (GVHD). Therefore, NK cells are promising candidates for the treatment of hematological malignancies.
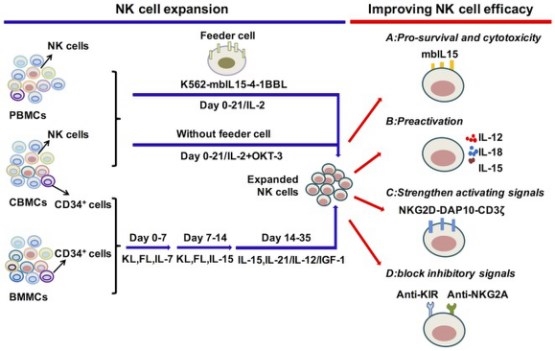
Fig.1 Cytokine regulation of NK cell expansion and cytotoxicity. (Wu, 2017)
The adoptive transfer of ex vivo expanded NK cells requires enhanced natural cytotoxicity and homing to tumor sites while maintaining "self" protection. There are several validated protocols for the expansion of NK cells ex vivo, all of which have specific characteristics and functions. At Creative Biolabs, we provide a comprehensive overview of the cytokine cocktails combination strategies and are committed to developing viable NK expansion strategies to meet the needs of customers. Currently, we provide the following ex vivo NK cell expansion protocols starting with purified NK cells (Fig.2) or PBMC (Fig.3):
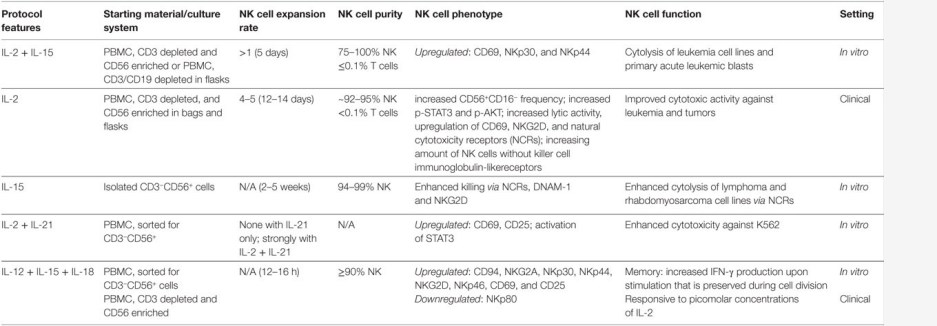
Fig.2 Ex vivo expansion of pure natural killer (NK) cells with cytokines starting with purified NK cells.
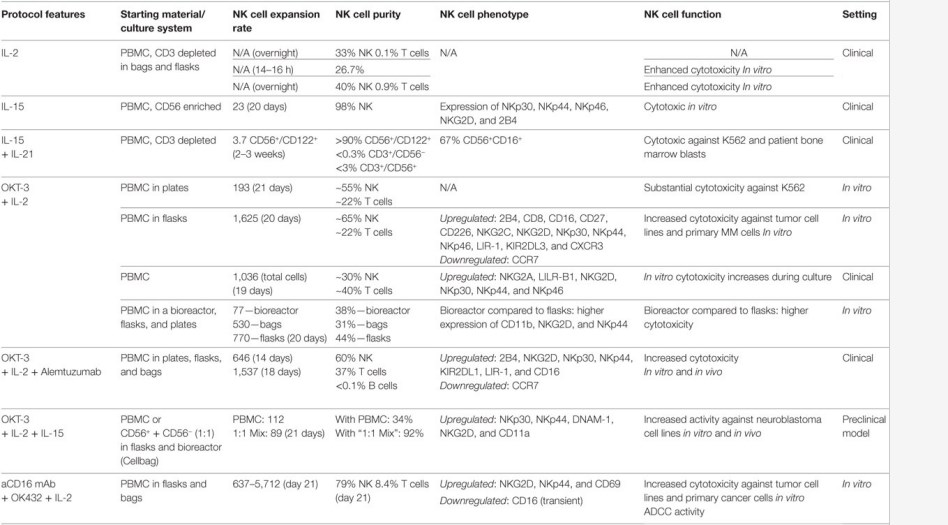
Fig.3 Ex vivo expansion of pure natural killer (NK) cells with cytokines starting with purified PBMCs.
If you are interested in our services, please send an email to contact us, and our team will get back to you as soon as possible.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION