All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Neutralizing antibody (NAb) is responsible for blocking the entry of a pathogen into a cell. Creative Biolabs provides human leukocyte antigen (HLA) neutralizing assay to assess the neutralizing activity of HLA antibodies against virus-carrying HLA alleles.
NAb is an antibody that is responsible for defending cells from pathogens. As part of the immune response, pathogens are triggered by both infections and vaccinations against infections. Neutralizing antibodies can result in lifelong immunity to certain infections and can be used to see if a person has developed immunity to an infection after they have recovered from it.
At present, many antibody therapies based on neutralization mechanisms have emerged for the treatment of various diseases including but not limited to infectious diseases and endocrine disorders. The neutralizing assays are specially designed to routinely validate and measure the therapeutic action of such antibodies. The role of neutralizing antibodies is as follows:
Creative Biolabs offers HLA antibody neutralizing assays that are specifically designed to measure antibody neutralization potential against different targets, including but not limited to virus, cytokine, and toxin.
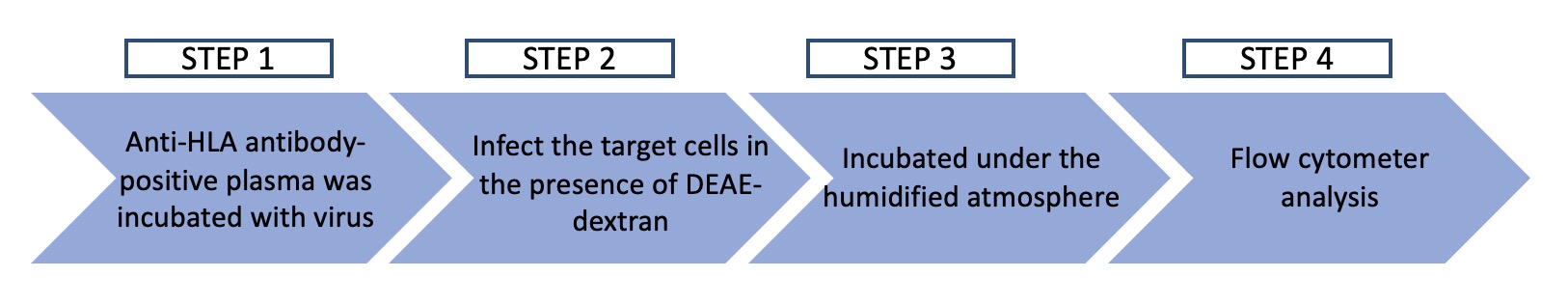 Fig.1 Workflow of HLA neutralizing assay.
Fig.1 Workflow of HLA neutralizing assay.
Except for HLA neutralizing assay, Creative Biolabs offers a complete range portfolio of HLA testing assays. For more information, please feel free to contact us directly.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION