All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Currently, CAR-T therapy using ex vivo-generated cells poses multiple barriers associated, being complicated and expensive to manufacture, and with a high risk of toxicity. Therefore, in vivo CAR-T Cell Engineering is a potential solution with the ease of production and the possibility of standardization. Furthermore, studies have shown that in vivo CAR-T Engineering is associated with a reduced risk of the most common toxicities, such as CRS, and "on-target, off-tumor" toxicity.
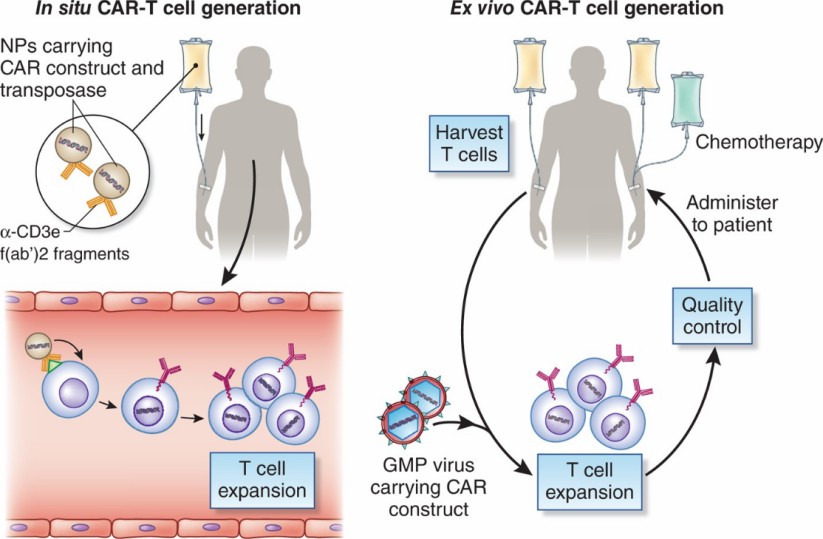 Fig.1 CAR-T-cell generation in situ or ex vivo. (Olweus, 2017)
Fig.1 CAR-T-cell generation in situ or ex vivo. (Olweus, 2017)
The rapid development of non-viral delivery systems has brought new perspectives to the design of in vivo CAR-T cell engineering. The non-viral delivery system has many advantages, its processing can take a variety of optimized formulations, and its synthesis is more streamlined, enabling precise drug delivery, reducing toxicity, and increasing the targeting of in vivo CAR-T therapeutics. Powered by years of experience in the CAR-T field, Creative Biolabs possesses strong capabilities in providing in vivo non-viral CAR-T Cell engineering services to facilitate customers' projects. There are some of our featured services as follows for reference, meanwhile, we also provide highly-customized in vivo non-viral CAR-T Cell engineering services to help global customers.
We provide one-stop in vivo non-viral CAR-T cell engineering services to assist clients' projects, here are several prominent services you can refer to:
Here are some results displaying the in vivo non-viral CAR-T cell engineering as a potentially ideal solution for tumor immunotherapy.
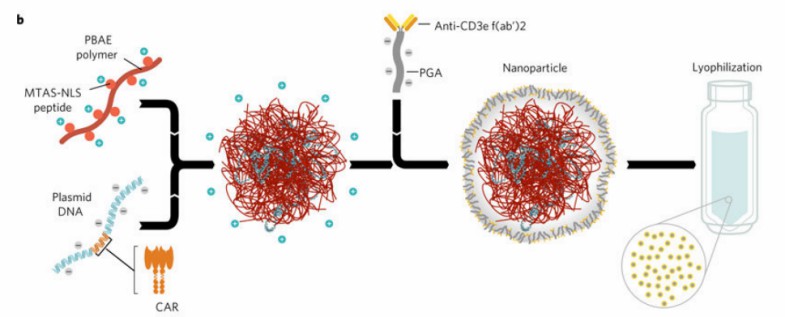
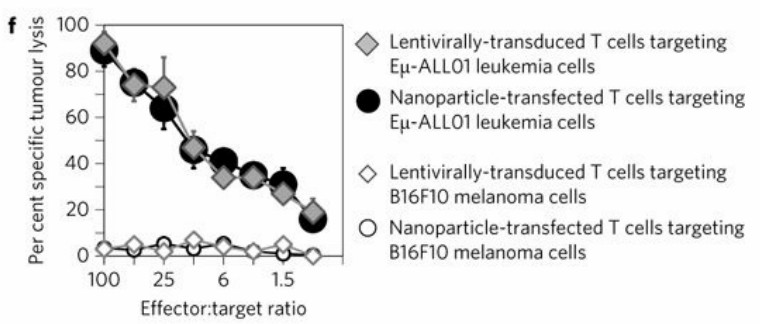
| 1. In situ CAR-T programming using synthetic DNA nanocarriers. | |
|
|
| Fig.2 Design and manufacture of DNA nanocarriers for in vivo CAR-T programming. (Smith, et al., 2017) | Fig.3 In vitro cytotoxicity assay of nanoparticle-transfected T cells against leukemia cells. (Smith, et al., 2017) |
| 2. In situ CAR-T programming using IVT mRNA nanocarriers. | |
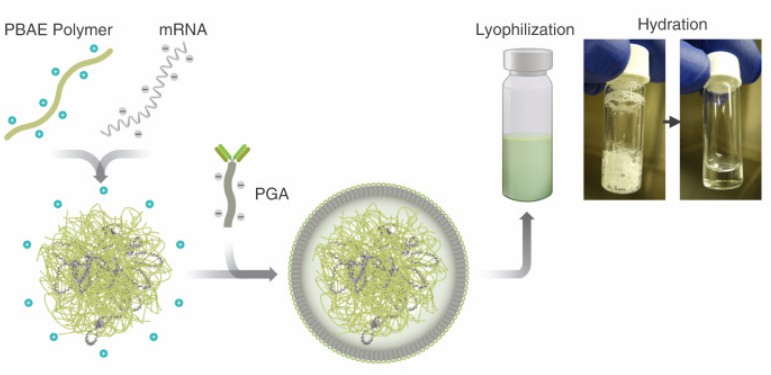
|
|
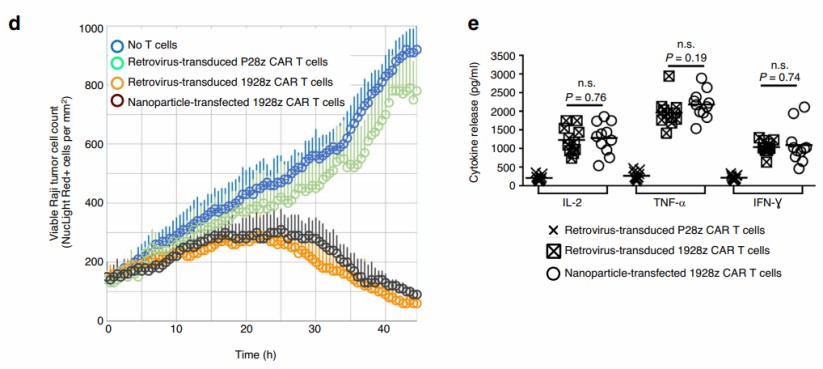
| Fig.4 Design and manufacture of IVT mRNA nanocarriers for in vivo CAR-T programming. (Parayath, et al., 2020) | Fig.5 In vitro cytotoxicity assay of nanoparticle-transfected T cells against Raji lymphoma cells. (Parayath, et al., 2020) |
Q1. Whether in vivo non-viral CAR engineering solution can be applied to other cells?
A: In vivo non-viral CAR-T cell engineering has many potential directions of application, such as in situ CAR-NK cells programming using the non-viral delivery system, in suit CAR-M cells programming.
Q2. What are the advantages of the non-viral approach comparing the viral-based approach for in vivo CAR-T engineering?
| Types of Vector | Pros | Cons | |
| Non-viral vector | Lipid-based nanoparticles | Simple manufacturing process | Low transfection efficiency |
| Polymer-based nanoparticles | High genetic cargo capacity | Toxicity related to materials | |
| Inorganic nanoparticles | Low immunogenicity | ||
| Viral vector | Adenovirus | High transfection | Limited genetic cargo capability |
| Retrovirus | efficiency | Immunogenicity | |
| Lentivirus | High stability of expression | Host genome integration | |
Professional consultation provides you with guidance throughout the entire project process, please contact us for a tailored solution.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION