All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Metastatic castration-resistant prostate cancer (mCRPC) is the late stage of prostate cancer. Researchers found that STEAP1, a six transmembrane epithelial antigen of the prostate 1, is highly expressed on prostate tumor cells and presents a more frequent and homogeneous expression in advanced prostate tumors. An anti-STEAP1 CAR-T cell is developed and shows specific killing with metastatic prostate cancer cells and prolonged survival of STEAP1+mice after adoptive transfer. Combined with collagen binding domain (CBD)-IL-12 therapy, STEAP1 CAR-T is promising to eradicate tumor cells with enhanced antigen presentation.
Metastatic castration-resistant prostate cancer (mCRPC) is a late-stage prostate cancer with high malignant, incurable, and short median survival time. Due to the resistance to approved ablation of androgen receptor (AR) signaling pathway-based therapies, current clinical treatments can only prolong patients' life for some months. Scientists have developed PSMA-targeted CAR-T cells for prostate cancer, which are safe and responsive, but the therapeutic efficacy is limited for the heterogeneous expression in mCRPC.
Previously, researchers from this article found that STEAP1, a six transmembrane epithelial antigen of the prostate 1, is highly expressed on prostate cancer cells using transcriptomic and proteomic techniques. Recently, they analyzed the expression landscape of STEAP1 in over 120 metastatic tumor tissues from forty-four mCRPC patients and demonstrated that STEAP1 presents a more frequent and more homogeneous expression in advanced prostate tumors.
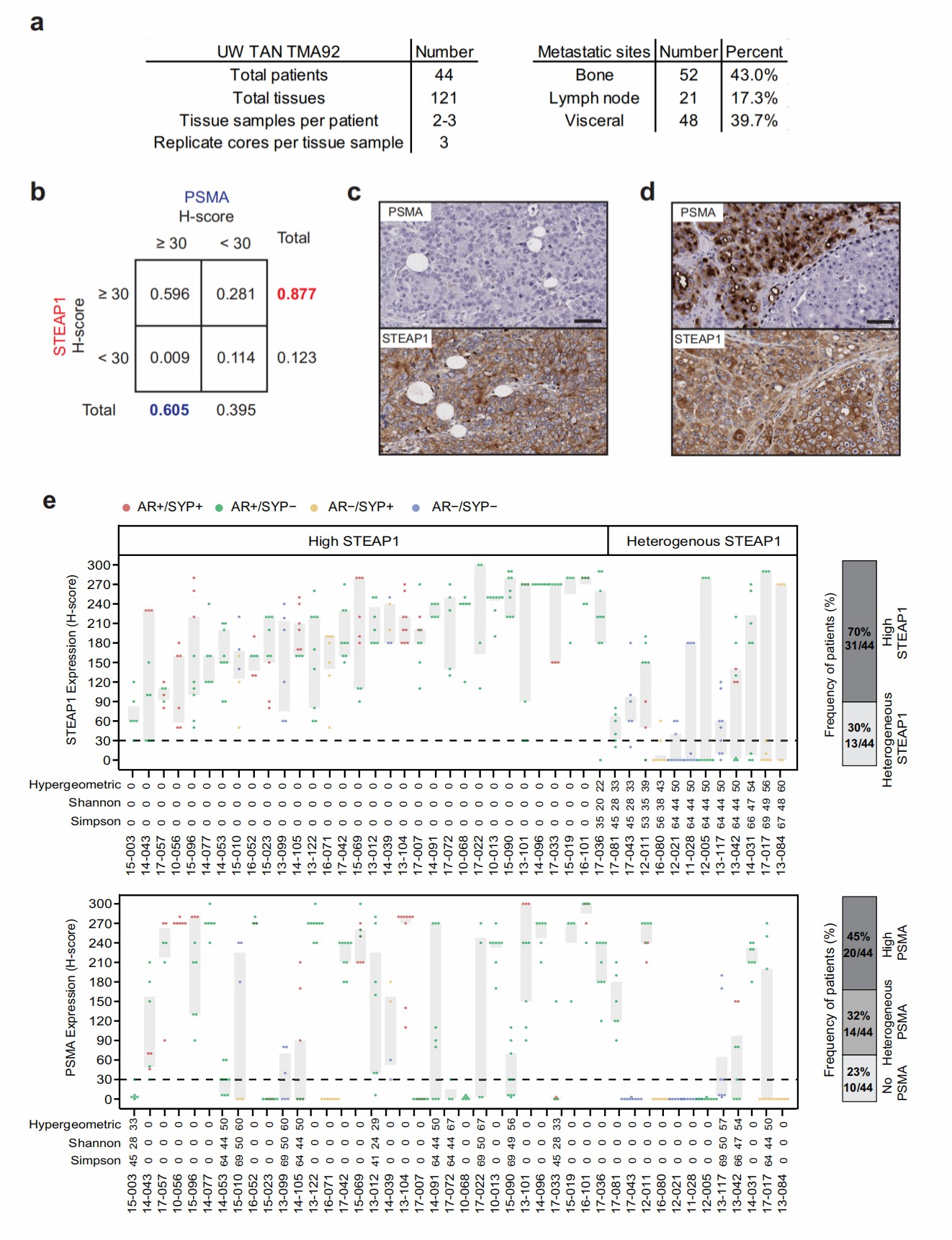 Fig.1 Characterization of STEAP1 and PSMA expression profile in mCRPC patients using immunohistochemistry.1
Fig.1 Characterization of STEAP1 and PSMA expression profile in mCRPC patients using immunohistochemistry.1
Broad expression in cancer and limited presentation in normal tissue makes STEAP1 an attractive antigen for targeted immunotherapy. Anti-STEAP1 CAR-T cells are generated using a lentiviral vector that contains a specific scFv, a CD28 transmembrane domain, and a 4-1BB co-stimulatory domain. These CAR-T cells specifically recognize human STEAP1 protein and do not cross-react with mouse Steap1 and other proteins in the human STEAP family. STEAP1-CAR also showed strong antitumor effects in the STEAP1 knock-in mouse model. To increase the therapeutic results of STEAP1 CAR-T treatment of mCRCS, collagen-binding domain IL-12 (CBD-IL-12) is injected simultaneously to remodel tumor immunosuppressive microenvironment and improved antigen processing and presentation.
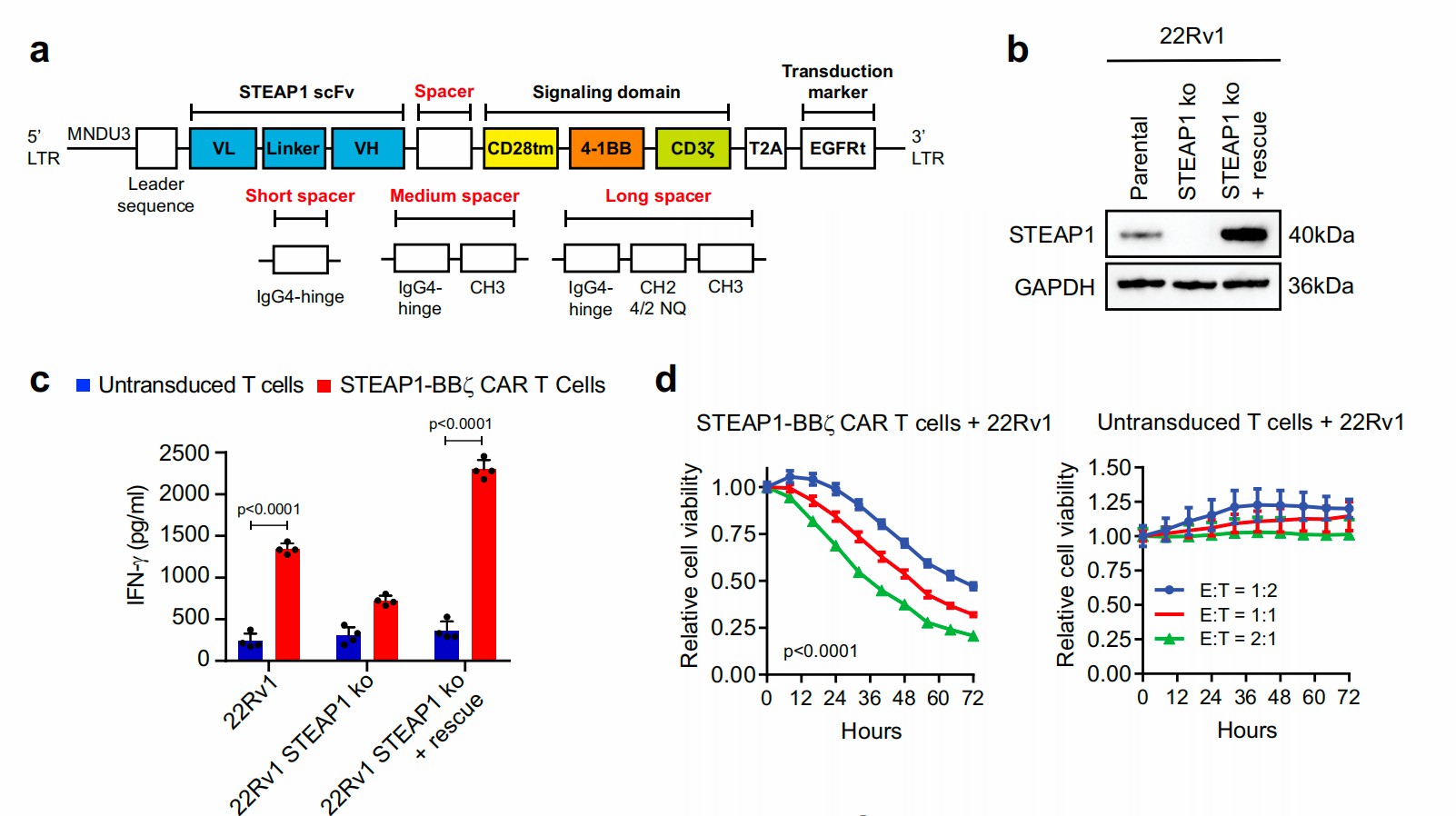 Fig.2 Anti-STEAP1 CAR-T generated by lentiviral transfection and showed antigen-specific cytotoxicity.1
Fig.2 Anti-STEAP1 CAR-T generated by lentiviral transfection and showed antigen-specific cytotoxicity.1
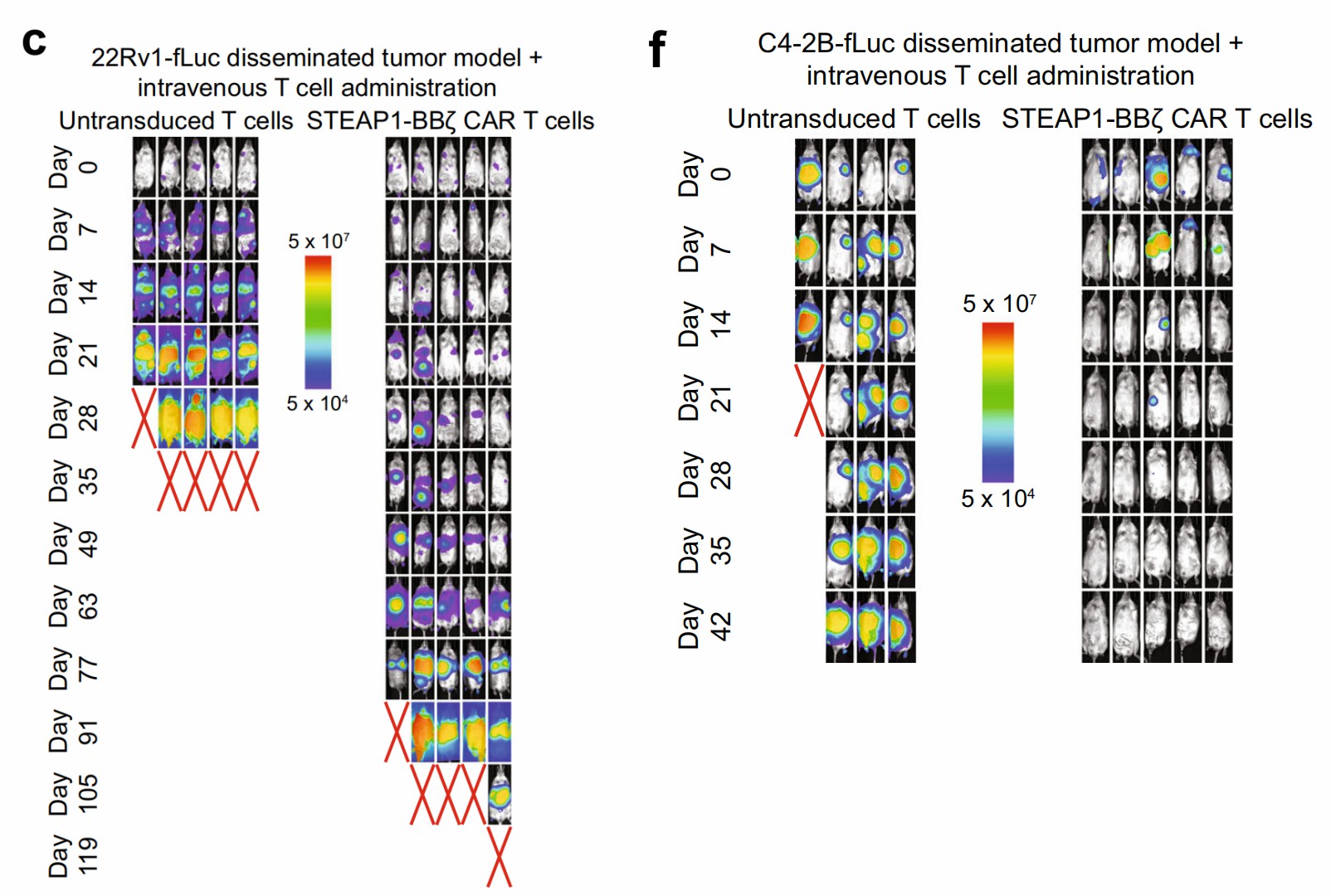 Fig.3 STEAP1 CAR-T cells displayed significant anti-tumor function in mouse models.1
Fig.3 STEAP1 CAR-T cells displayed significant anti-tumor function in mouse models.1
CAR-T cell therapy is exceptional in blood cancer treatment and has promising potency in solid tumors. Creative Biolabs is experienced in cell therapeutics and has successfully helped our clients develop a series of effective CAR-immune cell products. With cutting-edge methods and professional experts, we provide valuable strategies to engineer your target immune cells and accelerate your success.
Biomarker Identification & Selection
Cancer biomarker is used for diagnosis, prognosis, and targeted treatment. We provide a wide range of biomarkers covering most identified cancer targets for CAR-T efficacy tests. We also offer advanced novel tumor-associated antigen discovery services in customized situations.
An scFv contains the heavy chain and light chain of a monoclonal antibody. A superior scfv domain is a key factor for CAR-T function and efficacy. Creative Biolabs can help produce scFv from hybridoma cell lines and phage display. We also provide scFv characterization service and a large CAR-library screening service for high-affinity CARs.
Chimeric antigen receptor (CAR) is an artificial T cell receptor. The classic CAR construct contains an antigen binding domain, a hinge spacer, a transmembrane domain, a co-stimulatory, and CD3 ζ. Creative Biolabs provides four-generation CARs and special CARs for customized objectives.
CAR-T Gene Packaging & Delivery
CAR constructs are delivered to T cells to modify cell functions. Creative Biolabs offers multiple approaches to genetically modifying T cells. Retroviral transfection, lentiviral transfection, and electroporation are advanced techniques flexibly used by our scientists for CAR-T development.
Primary Mouse CAR-T Production
Creative Biolabs offers primary mouse CAR-T development services for clients to facilitate CAR-T in vivo function tests. We have an optimal production protocol with enhanced mouse T-cell transfection efficacy and elevated in vitro expansion capacity. Furthermore, we provide a complete validation of the mouse CAR-T before delivery.
CAR Cell In Vitro Assay Service
Creative Biolabs provides multiple evaluations on generated CAR-T cells on various aspects to verify phenotype and ensure functions. Our service includes car expression tests, cytokines secretion tests, in vitro cytotoxicity tests, cell proliferation tests, viability, and bio-distribution analysis.
Creative Biolabs is not only a lead cell therapy provider, but we also provide a wide range of cell therapy-related products to help clients manipulate immune cells with reliable and high-quality commercial kits and reagents. Here are some products used for CAR-T generation, hope you can find the proper one.
PSMA-Targeted Product Category
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION