All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
To date, CAR-T cell immunotherapy has received tremendous success for treating hematological malignancies (e.g., anti-CD19 CAR-T cells in leukemias), while not yet extrapolated to solid tumors. A major obstacle associated with current CAR-T cell therapy is that the limited replicative lifespan of CAR-T cells prohibits the in vivo expansion and long-term persistence of these cells, potentially hindering the long-term therapeutic efficacy of CAR-T cells. Many approaches have been evaluated such as adding signaling moieties from co-stimulatory molecules (the 2nd and 3rd generation of CAR), using T cell recognizing Epstein-Bar virus antigens and selecting central memory T cells for genetic modification.
However, CAR-T cell therapy may cause some acute on-target/on-tumor toxicity (cytokine release syndrome, tumor lysis syndrome) or on-target/off-tumor toxicity, and in some cases, CAR-T cells undergo antigen-independent proliferation that could increase cell-mediated toxicity and raise the concern of immortalization of infused CAR-T cells. Therefore, the 4th generation of CAR has been developed to address these problems, for example, harboring an inducible suicide gene in the CAR construct.
As a frontier biotech service provider, Creative Biolabs now provides preclinical viability and bio-distribution analysis services for CAR-T cell therapy. All tests are conducted by experienced technicians on the most appropriate models with the most advanced techniques.
Our studies focus on but not limited to the following aspects.
In Vivo Proliferation, Survival, and Long-term Persistence of CAR-T Cells
T Cell Migration and Distribution
Memory T Cell Response
T Cell Perseverance
The tumor microenvironment contains multiple inhibitory factors to potentially suppress CAR-T cells. Tumor cells also develop many mechanisms to avoid the eradication by CAR-T cells.
Efficacy of Next Generation CAR
Techniques Involved
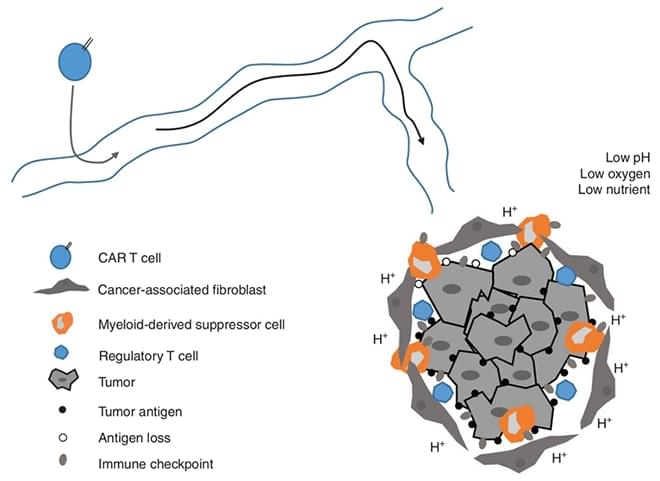
Immunosuppressive Tumor Microenvironment
Newick K et al. Mol Ther Oncolytics, 2016
All studies are performed in a GLP-compliant and IACUC-regulated facility. We are glad to share our valuable experience on the study of viability and bio-distribution of CAR-T cell with our clients. Scientists of Creative Biolabs will work with you to design the program that best fits your requirements and expedite your CAR-T therapy to clinical trials.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION