Cell-based Checkpoint Inhibitor Assay Service
Introduction Strategies What We Can Offer Workflow Highlights Publication Customer Reviews FAQs Extended Services
Cell-based Checkpoint Inhibitor Assay: Unlock New Therapeutic Potential!
The development of immune checkpoint inhibitors has revolutionized cancer therapy. These therapies target checkpoint proteins such as PD-1, PD-L1, and CTLA-4, which are critical in regulating the immune system's ability to recognize and attack cancer cells. Recent studies have shown that utilizing cell-based assays to evaluate checkpoint inhibition can greatly enhance the prediction of therapeutic efficacy and guide the development of novel cancer immunotherapies. With increasing reliance on immune checkpoint inhibitors, it is crucial for researchers to have effective tools that provide accurate, reproducible results.
Are you currently facing challenges in immuno-oncology drug discovery, such as identifying effective checkpoint inhibitors or understanding immune cell behavior? Creative Biolabs' cell-based checkpoint inhibitor assay helps you advance your research by enabling the evaluation of immune checkpoint interactions in a biologically relevant environment. Our assay technology provides precise insights into immune modulation, facilitating the development of more effective immunotherapies.
Here's what you can expect:
- Precise Inhibition Evaluation: Accurately assess checkpoint inhibitor efficacy by monitoring immune cell activation, cytokine production, and tumor cell apoptosis.
- Comprehensive Data: Obtain data on immune cell activation, co-stimulation, and cytokine responses, which is critical for designing the next generation of cancer immunotherapies.
- Expert Support: Our team provides consultation, assay optimization, and troubleshooting to ensure your research progresses smoothly.
Strategies
When developing cell-based checkpoint inhibitor assays, several strategies are crucial for achieving reliable results. These include:
1. Cell Line Selection: Choosing the right cell lines, such as tumor cell lines expressing specific checkpoint ligands or immune cells with receptor expression, is essential for mimicking the tumor microenvironment.
2. Optimizing Assay Conditions: Fine-tuning cytokine concentrations, cell density, and incubation times for consistent and reproducible assay outcomes.
3. Multiplexed Readouts: Using multiple readout technologies such as flow cytometry, ELISA, and reporter gene assays to capture a full spectrum of immune responses.
What We Can Offer
Creative Biolabs provides comprehensive solutions for evaluating immune checkpoint inhibitors, tailored to your research needs. Our advanced cell-based assays allow for:
- High Sensitivity: Detecting subtle changes in immune activation and checkpoint inhibition.
- Real-World Relevance: Mimicking human immune response in a controlled laboratory setting.
- Customizable Assays: Ability to adjust for specific checkpoint pathways, immune cell types, and cancer models.
We offer both ready-to-use kits and customizable assays, ensuring flexibility for a variety of research applications.
Discover How We Can Help - Request a Consultation
Workflow
1. Assay Setup & Optimization
Once the assay design is finalized, we begin the preparation phase. This includes culturing the selected cell lines, preparing reagents, and optimizing culture conditions such as cytokine concentrations, cell density, and incubation times. We also calibrate any necessary equipment, such as flow cytometers or ELISA readers, to ensure data accuracy.
2. Immune Checkpoint Inhibitor Treatment
At this stage, the assay is run by treating the cells with your chosen checkpoint inhibitors. This is followed by a series of immune response assays to evaluate how the inhibitor modulates immune cell activation, tumor cell apoptosis, and checkpoint blockade. In addition to monitoring immune cells, we'll track cytokine release and other immune markers to assess the effectiveness of the treatment.
3. Data Collection & Analysis
Once the assay has been completed, data is collected from various readout technologies such as flow cytometry (to measure cell surface markers), reporter gene assays (for activity measurement), and cytokine assays (for immune activation). Our team compiles the data to offer both qualitative and quantitative insights into the checkpoint inhibition effectiveness.
4. Final Report & Recommendations
After analyzing the data, Creative Biolabs provides a comprehensive report detailing the results of your assay, including immune activation profiles, efficacy of checkpoint inhibitors, and potential next steps. If necessary, we also provide recommendations for follow-up experiments, additional checkpoints to explore, or refinement of the assay protocol for better optimization.
Required Starting Materials:
- Cell Line Information: Provide the tumor or immune cell lines that express the specific checkpoint ligands/receptors of interest (e.g., PD-L1, PD-1, CTLA-4).
- Checkpoint Inhibitor Information: Specify the checkpoint inhibitors to be tested, including monoclonal antibodies or small molecules targeting immune checkpoints.
- Research Objective: Outline the therapeutic target or pathway you are investigating, helping our team tailor the assay for maximum relevance to your project.
Highlights
Real-World Relevance
Unlike traditional biochemical assays, our cell-based assays simulate the actual immune response in vivo. This allows for a more accurate prediction of how checkpoint inhibitors will perform in human clinical settings. By mimicking the tumor microenvironment, our assays provide data that is more predictive of therapeutic outcomes, giving you a competitive edge in drug development.
Customization for Your Needs
We understand that every research project is unique. That’s why our assays are fully customizable. Whether you need to assess immune checkpoint interactions in specific cancer types or optimize the use of a novel checkpoint inhibitor, we work with you to create assays that meet your exact research requirements. This flexibility ensures that the assays are relevant and impactful for your study.

High Sensitivity and Precision
Our assays are highly sensitive, capable of detecting even subtle changes in immune cell activity, cytokine secretion, and tumor cell apoptosis. This allows you to identify potentially effective checkpoint inhibitors at early stages of development. The precision of the assay ensures that you can reliably interpret small variations in your results, which are crucial for optimizing therapeutic strategies.
Expert Consultation & Support
In addition to providing cutting-edge assay technology, Creative Biolabs offers expert consultation at every stage of your project. Whether you need help selecting the right immune checkpoint targets or optimizing assay conditions, our team is here to assist you. We work with you to ensure that you are getting the most out of your experiments and that your results are both actionable and reproducible.
Experience the Creative Biolabs Advantage - Get a Quote Today
Publication
The CH-4 analog is a small molecule developed to specifically target the PD-1/PD-L1 interaction, a key immune checkpoint pathway in cancer immunotherapy. In vitro studies demonstrate that this compound effectively inhibits the binding between PD-1 on T cells and PD-L1 on tumor cells, thereby promoting T cell activation and immune response against cancer cells. The research showcases the compound's ability to block the PD-1/PD-L1 axis with high potency, highlighting its potential as a novel therapeutic approach in cancer treatment. This inhibition is crucial for enhancing anti-tumor immunity, offering promising therapeutic implications for the development of immune checkpoint inhibitors.
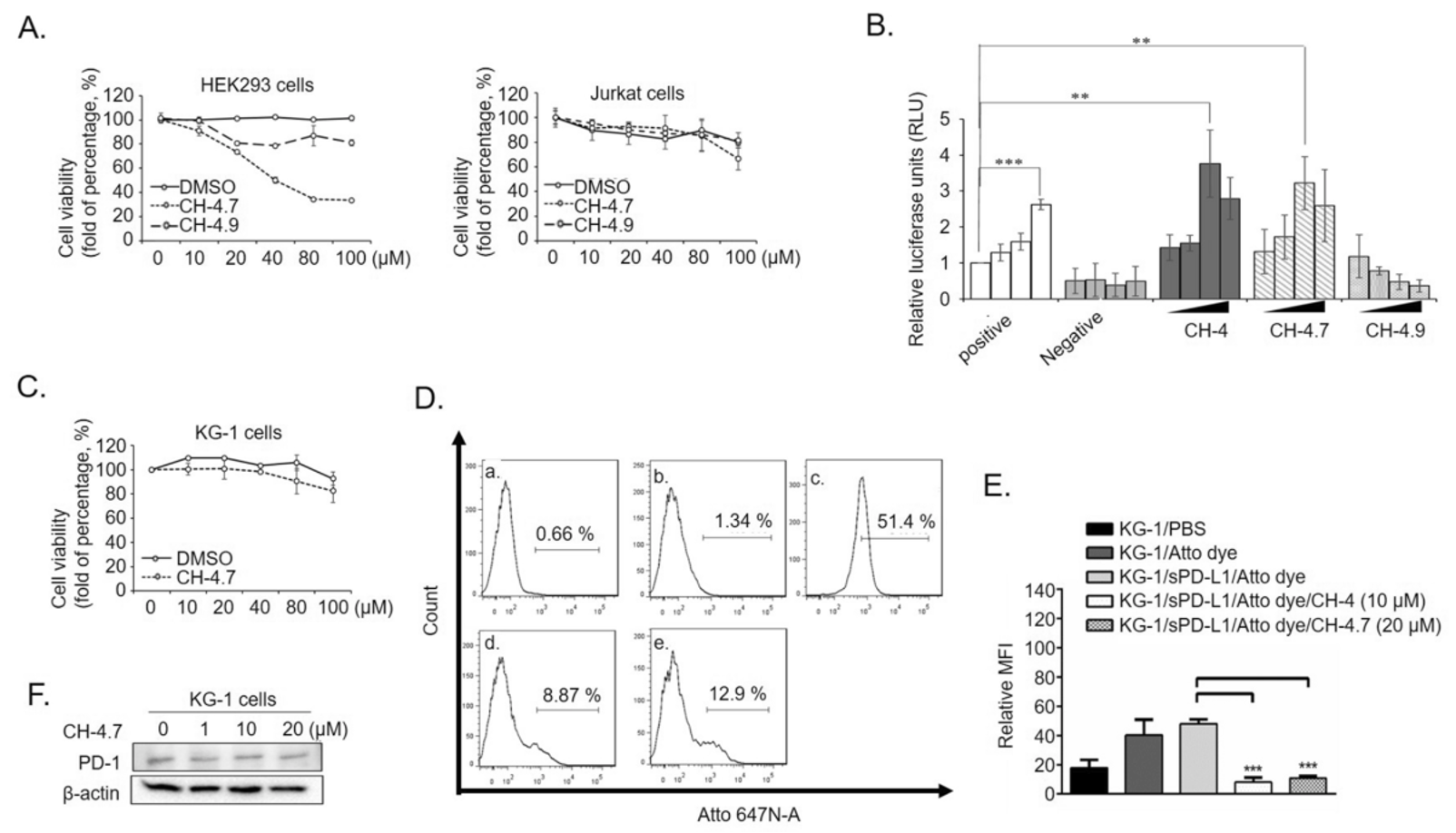 Fig.1 The CH-4 analog effectively inhibited the PD-1/PD-L1 interaction in vitro, a critical pathway targeted in human lung cancer cell A-427 to enhance anti-tumor immunity.1
Fig.1 The CH-4 analog effectively inhibited the PD-1/PD-L1 interaction in vitro, a critical pathway targeted in human lung cancer cell A-427 to enhance anti-tumor immunity.1
Customer Reviews
1. Precise Inhibition Evaluation: Using Creative Biolabs' Cell-based Checkpoint Inhibitor Assay in our research has significantly improved our ability to assess PD-1/PD-L1 interactions in vitro. The assay's sensitivity allowed us to detect subtle inhibitor effects, guiding our development of more effective therapeutics. *June 2025, Dr. J**do.
2. High Sensitivity and Reproducibility: The assay platform's reproducibility is impressive. We have successfully used it to evaluate multiple checkpoint inhibitors across various immune cell types, leading to more informed decisions in our drug development pipeline. *May 2025, Dr. M**son.
3. Excellent Support and Results: The team at Creative Biolabs provided expert guidance in customizing the assay to our specific cancer model, which helped us advance our checkpoint inhibitor testing. The results were reliable and aligned with our clinical predictions. *April 2025, Dr. P**son.
FAQs
How do I choose the right cell line for my checkpoint inhibitor assay?
Selecting the appropriate cell line depends on the target checkpoint and the immune cells involved. Our team can assist you in choosing based on your research objectives.
Can I use your assays for testing novel checkpoint inhibitors?
Yes, our assays are fully customizable to accommodate new checkpoint inhibitors or immune-modulatory compounds.
What kind of readout technologies do you use for data analysis?
We employ a range of technologies, including flow cytometry, ELISA, and reporter gene assays, to capture comprehensive immune responses.
What are the advantages of using cell-based assays over other in vitro methods?
Cell-based assays closely mimic the human immune system, offering more accurate and relevant data for therapeutic development.
Extended Services
In addition to our Cell-based Checkpoint Inhibitor Assay, Creative Biolabs offers a variety of related services to complement your immuno-oncology research:
-
Custom Cell Line Development: Tailored cell lines expressing specific checkpoint proteins or immune cell markers.
-
Immune Response Profiling: A suite of assays to analyze cytokine profiles, T cell activation, and immune modulation.
-
Immune Cell Isolation & Characterization
We offer services for isolating and characterizing specific immune cell populations, such as CD8+ T cells, NK cells, and dendritic cells. These immune cells can be used to create in vitro models for studying immune checkpoint inhibitors and their effect on immune responses in cancer therapy.
- Cell Isolation: Isolate high-purity populations of T cells, B cells, or NK cells from peripheral blood or tumor biopsies.
- Cell Characterization: Perform phenotypic analysis and functional assays to evaluate immune cell behavior in response to checkpoint inhibitors.
Contact our team today for more information about our cell-based checkpoint inhibitor assay and how we can support your research. Request a consultation now and let us help you advance your immuno-oncology projects!
Reference
-
Lu, Chih-Hao, et al. "In vitro characterization of a small molecule PD-1 inhibitor that targets the PD-l/PD-L1 interaction." Scientific reports 12.1 (2022): 303. DOI: 10.1038/s41598-021-03590-4. Distributed under Open Access license CC BY 4.0, without modification.



 Fig.1 The CH-4 analog effectively inhibited the PD-1/PD-L1 interaction in vitro, a critical pathway targeted in human lung cancer cell A-427 to enhance anti-tumor immunity.1
Fig.1 The CH-4 analog effectively inhibited the PD-1/PD-L1 interaction in vitro, a critical pathway targeted in human lung cancer cell A-427 to enhance anti-tumor immunity.1
