Immunogenicity Prediction Services
Overview Publication Why Choose Us FAQs Customer Review Related Services Contact Us
Overview
Immunogenicity is the most critical safety issue associated with biologics. Immunogenicity is the ability to induce humoral and/or cell-mediated immune responses. Most biologics induce immune responses because they are polypeptides or proteins and thus can be recognized by the immune system as foreign objects. Given that biologics may induce this unwanted immune response; it is important to study the immunogenicity of biological agents prior to marketing approval.
To help you understand and address the potential issues of drug immunogenicity in your program at any stage, Creative Biolabs has developed Sensitive Immunogenicity Assessment Technology (SIAT) according to specific and EMA guidelines. SIAT-based highly sensitive and unique assessment techniques include in silico assessment and in vitro screening, which are effective in a wide range of applications, including biopharmaceutical quantification, anti-drug antibody (ADAs) assays, and pharmacokinetic / pharmacodynamics efficacy measurements.
In Silico Assessment - T Cell Epitope Screening
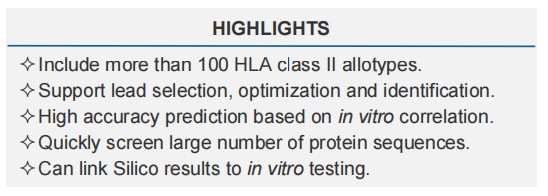 Driven by sequencing technology, structural bioinformatics and experimental data analysis, Creative Biolabs has developed the SIAT-based in silico assessment technology, a proprietary T cell epitope screening tool for identifying potential epitopes in biotherapeutic proteins and new antibody targets. The in silico tool predicts potential peptide/HLA binding by mimicking the structural features of the MHC class II and experimentally determines binding affinity, which are prerequisites for T cell activation.
Driven by sequencing technology, structural bioinformatics and experimental data analysis, Creative Biolabs has developed the SIAT-based in silico assessment technology, a proprietary T cell epitope screening tool for identifying potential epitopes in biotherapeutic proteins and new antibody targets. The in silico tool predicts potential peptide/HLA binding by mimicking the structural features of the MHC class II and experimentally determines binding affinity, which are prerequisites for T cell activation.
In Vitro Screening
Creative Biolabs's in vitro platform assesses the immunogenicity risk of biologics by directly measuring immune responses using peripheral blood mononuclear cells (PBMCs). When combined with our in Silico tool, these cell analyses allow for a complete immunogenic risk assessment of your primary lead candidate. In vitro assays include: 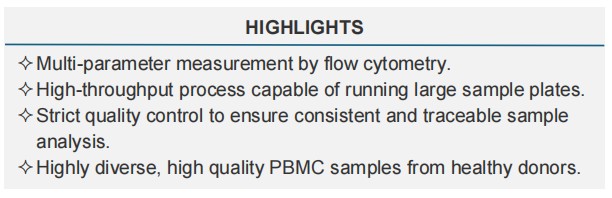
In Vivo Screening
In vitro and ex vivo assays provide a direct preview of the immunogenicity of biotherapeutic drug candidates, but these assays have some limitations. Creative Biolabs also offers a one-stop in vivo immunogenicity assessment service, which uses a variety of animal models, including HLA transgenic mice and humanized mice, to provide the most direct assessment of the molecules before entering clinical trials.
Creative Biolabs' SIAT-based in silico services and in vitro screening provide the most comprehensive immunogenicity assessment package, which addresses the challenges of unwanted immune responses throughout the drug development cycle. By managing potential drug immunogenicity at the earliest possible stage, you can save time and money while creating safer and more effective biotherapeutic drugs for the market.
Publication
This publication introduces AblmmPred, a new computational method for predicting the immunogenicity of therapeutic antibodies using features derived from the AntiBERTy pre-trained language model. The goal is to address the time-consuming and costly nature of traditional wet-lab experiments for assessing immunogenicity. AblmmPred was developed by training a machine learning model on a dataset of 199 commercial therapeutic antibodies, using only the amino acid sequences of their variable regions. The method avoids the need for 3D antibody structures, which are required by other methods like PITHA. The results show that AblmmPred outperforms a comparison method, PITHA, with an accuracy of 0.7273 on an independent test dataset, which is 9.09% higher than the comparison method's accuracy. The authors suggest that this new method could help speed up the development of antibody therapeutics.
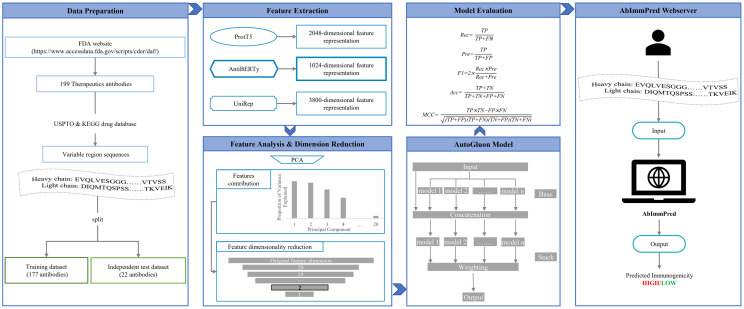 Fig.1 A step-by-step guide to the AbImmPred.1
Fig.1 A step-by-step guide to the AbImmPred.1
The Creative Biolabs Advantage in Immunogenicity Prediction
Creative Biolabs stands as a leader in immunogenicity prediction due to our two decades of specialized expertise, our integrated multi-platform approach, and a proven track record of de-risking biotherapeutic development. Our unwavering commitment to innovation, evidenced by our pioneering use of cutting-edge AI/ML models, ensures the highest accuracy and most insightful predictions available in the industry. Unlike traditional methods that may rely on cumbersome 3D structural data, our sequence-based, AI-driven approach offers superior efficiency and precision, as demonstrated by published data showing significant performance advantages over existing methods. Our extensive experience allows us to navigate the nuances of immunogenicity, providing tailored solutions that truly impact your project's success.
Ready to experience the Creative Biolabs advantage and accelerate your therapeutic development? Get a Quote Today and let us demonstrate our commitment to your success.
Your Questions Answered
Q1: Can Creative Biolabs' service predict immunogenicity for non-antibody biologics, such as fusion proteins or enzymes?
A1: Creative Biolabs specializes in antibody immunogenicity, but our platforms are adaptable to a wide range of protein therapeutics, including enzymes, fusion proteins, and peptides. Our experts can create a tailored prediction strategy for your specific biologic.
Q2: What actionable steps can I take if my therapeutic candidate shows high predicted immunogenicity through your service?
A2: Identifying high immunogenicity early is the core value of our service. Creative Biolabs' experts will collaborate with you to interpret high-risk profiles and provide actionable recommendations, such as epitope engineering or strategic sequence modifications, to guide your optimization efforts effectively.
Q3: Is it possible to obtain a quantitative measure of immunogenicity, rather than just a high/low classification, from Creative Biolabs?
A3: Our platform's primary output is a high/low immunogenicity classification for early-stage screening. We are continuously advancing our models and can discuss more nuanced, semi-quantitative assessments for projects with specific requirements.
Customer Reviews: Real-World Impact
-
Critical Early Insights
Creative Biolabs' immunogenicity prediction service provided critical early insights into our novel protein therapeutic. Their detailed report helped us identify a potential immunogenic region we hadn't considered, allowing us to pivot our engineering strategy before significant investment, saving us months of development time. - Dr. L****n
-
Comprehensive Validation
The combination of in silico predictions with their robust in vitro validation assays gave us a truly comprehensive understanding of our lead candidate's immunogenic profile. This dual approach from Creative Biolabs was essential for our regulatory submission, providing the solid data package we needed. - M. C***z
Complementary Biologic Development Services
To further support and accelerate your biotherapeutic development journey, Creative Biolabs offers a comprehensive suite of complementary services that seamlessly integrate with our immunogenicity prediction offerings:
Antibody Humanization Service
Humanized antibodies, which are modified from non-human species to resemble human antibodies, are crucial for therapeutic applications. They are designed to reduce the immunogenicity observed with murine antibodies in treatments like cancer immunotherapy. Creative Biolabs provides customized antibody humanization services using a variety of methods and toolkits.
Learn More →
T Cell Activation Analysis
Creative Biolabs offers optimal and customized solutions for T-cell activation analysis. Their flexible services and multiple methods help researchers quickly identify T-cell activation status to advance their projects.
Learn More →
Contact Us
Our labs are designed to meet the large-scale bioanalysis for your project, and our dedicated biologics team works with you to tailor your assessment strategy to your treatment goals and schedule using a full suite of immunogenicity assessment packages.
To obtain further information, please contact us.
Reference
-
Wang, Hong, et al. "AbImmPred: An immunogenicity prediction method for therapeutic antibodies using AntiBERTy-based sequence features." Plos one 19.2 (2024): e0296737. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.1371/journal.pone.0296737




 Fig.1 A step-by-step guide to the AbImmPred.1
Fig.1 A step-by-step guide to the AbImmPred.1
