Targeted Immuno-Stimulatory Conjugate Development Service
Introduction Workflow Why Choose Us What We Can Offer FAQs Customer Reviews Extended Services
Introduction
In developing targeted immuno-stimulatory antibody conjugates, choosing the right payload is equally important. Creative Biolabs relies on both data and experience to pick stimulatory agents—like cytokines or TLR agonists—that can reliably activate a meaningful immune response without overwhelming the system. To round out the process, every construct is put through thorough lab testing. We evaluate how it behaves in cells and animal models, paying close attention to immune activity, safety markers, and how the compound moves through the body. This gives us a clear picture of its potential before it moves forward.
Workflow
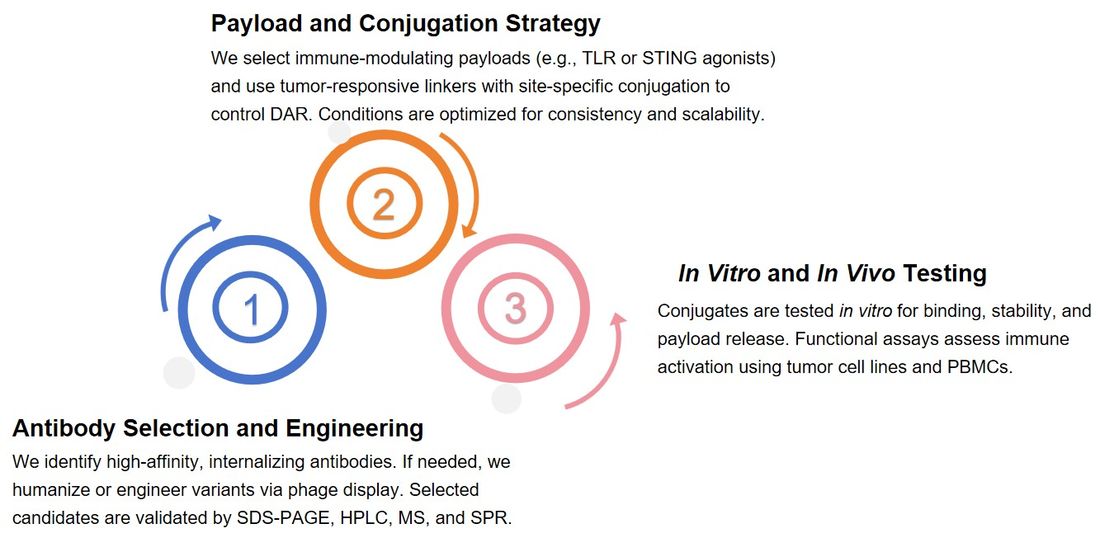
-
Antibody Selection and Engineering
We begin by identifying antibodies with strong target specificity, internalization ability, and binding affinity. When needed, we engineer or humanize antibodies and generate variants using phage display. Selected candidates are rigorously characterized using analytical methods such as SDS-PAGE, HPLC, mass spectrometry, and SPR.
-
Payload and Conjugation Strategy
Payloads—such as TLR agonists, cytokines, or STING agonists—are chosen based on the desired immune response. We design cleavable, stable linkers tailored to the tumor environment and apply site-specific conjugation to control DAR. Reaction conditions are optimized for consistent results and scalability.
-
In Vitro and In Vivo Testing
Conjugates are tested in vitro for binding, stability, and payload release. Functional assays assess immune activation using tumor cell lines and PBMCs.
Why Choose Us?
Extensive Scientific Expertise: Our team consists of highly skilled scientists with a strong background in antibody design, payload integration, and the development of immune-based therapeutics.
Advanced Conjugation Platforms: We implement leading-edge technologies for precise conjugation and offer access to a diverse portfolio of immune-activating payloads.
Client-Centered Customization: Our solutions are carefully crafted to align with each client's distinct project goals, ensuring the highest level of effectiveness.
Full-Spectrum Development Support: From initial concept to final validation, we deliver continuous guidance and technical support throughout the entire development journey.
What Can We offer
Antibody selection and engineering
Payload selection and linker design
FAQs
How do you ensure the stability and assess the safety and efficacy of the targeted immuno-stimulatory antibody conjugate?
-
We utilize advanced conjugation chemistry and linker technologies to ensure the stability of the targeted immuno-stimulatory antibody conjugate during circulation and its efficient release within the tumor microenvironment.
-
We conduct rigorous in vitro and in vivo studies, including toxicology assessments, pharmacokinetic/pharmacodynamic studies, and efficacy evaluations in relevant animal models.
How about intellectual property?
-
We work closely with our clients to ensure that their intellectual property is protected throughout the development process.
Customer Reviews
-
"The comprehensive support and expertise provided by significantly accelerated our targeted immuno-stimulatory antibody conjugate development program. The in vivo efficacy data was particularly compelling." [3 Years], J***s.
-
"Our advanced conjugation technology allowed us to achieve a highly defined DAR, which was critical for optimizing our targeted immuno-stimulatory antibody conjugate's therapeutic window." [2 Years], M***e.
-
"Using targeted immuno-stimulatory antibody conjugate service at Creative Biolabs, we were able to overcome challenges related to payload stability and achieve successful preclinical validation." [1 Year], D***d.
Extended Services
Contact our team for more information on targeted immuno-stimulatory antibody conjugates.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure