Co-inhibitory Immune Checkpoint Pathways
The success of cancer immunotherapy has generated tremendous interest in identifying new immunotherapeutic targets. Inhibition of immune checkpoint proteins can reduce the ability of the tumor microenvironment to suppress host antitumor immunity. Immune checkpoint inhibitor has been one of the most significant advances in the anticancer therapy in the past decade. Many immune checkpoint inhibitors have already shown remarkable clinical efficacy in various types of cancers. Co-inhibitory immune checkpoint pathways have been summarized as follows:
-
1. Programmed cell death-1 (PD-1)
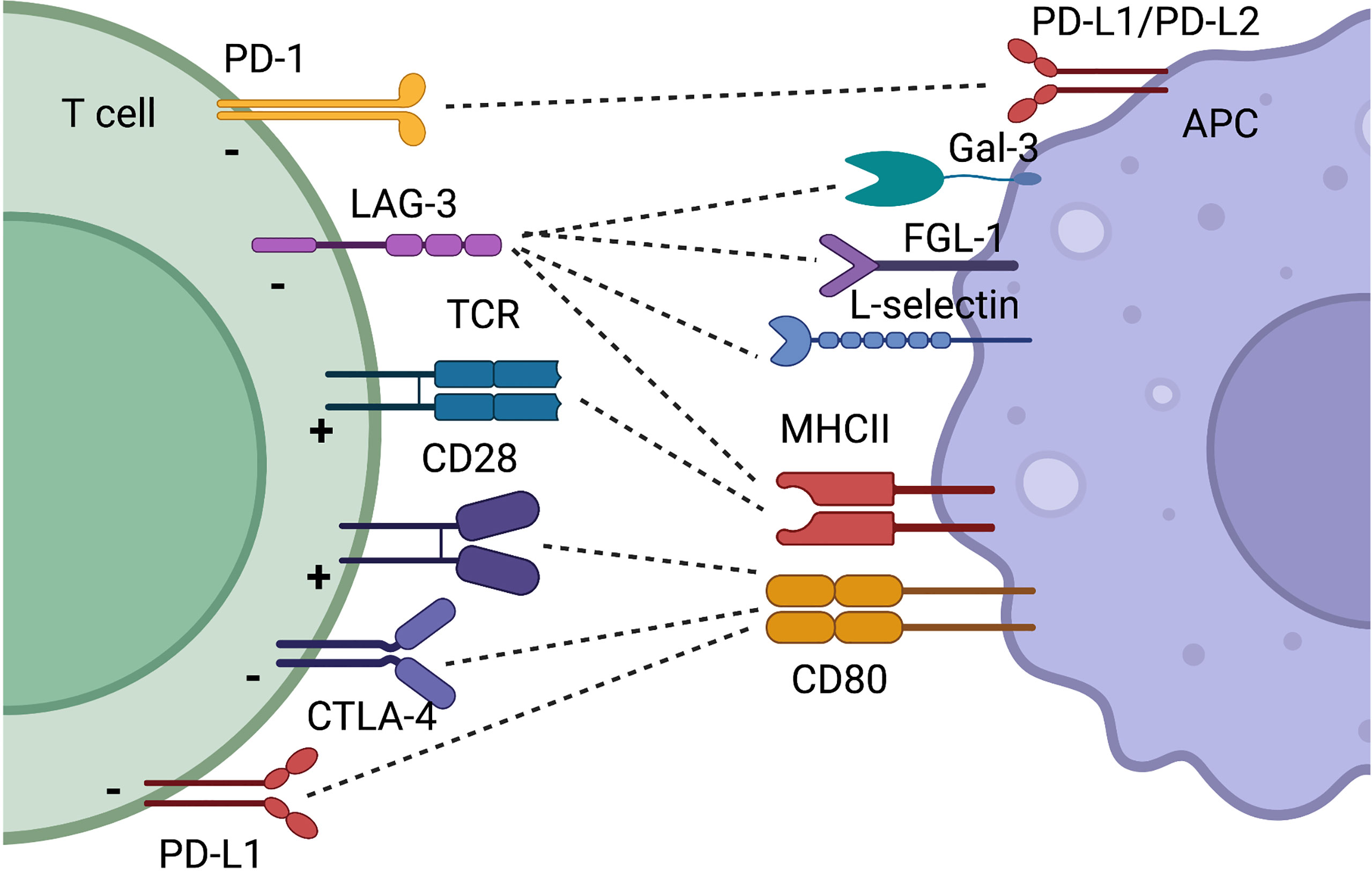
PD-1, also known as CD279, is a transmembrane protein expressed on the surface of circulating T-cells, B-cells and NK-cells, and is used to recognize “self” antigens from “non-self”. PD-1 pathway blockade has impressive clinical trial results with 30%- 50% response rates in a broad range of tumors.
PD-L1 is an inhibitory ligand, also known as B7-H1 or CD274, expressed on various cancer cells. The binding of PD-L1 to PD-1 receptors on immune effector cells inhibits the immune effector cells from attacking the tumor cells. PD-L2, also known as B7-DC or CD273, is the other known ligand of PD-1. PD-L2 is a cell surface protein expressed by activated macrophages and dendritic cells that binds PD-1 on T-cells to inhibit immune responses. The interaction of PD-L2 with PD-1 on T-cells regulates the balance between T-cell activation, tolerance, and immunopathology.
-
2. Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) & CD80/CD86
CTLA-4, also known as CD152, is expressed exclusively on T-cells, where it primarily regulates the amplitude of the early stages of T-cell activation. CTLA4 is the first immune checkpoint receptor to be clinically targeted. CTLA-4 blockade results in a broad enhancement of immune responses dependent on helper T-cells, and conversely, CTLA-4 engagement on regulatory T-cells (Tregs) enhances their suppressive function. CTLA-4 is highly activated on Tregs in a Foxp3-dependent manner and is a ligand for CD80 and CD86 expressed on APC. The interaction of CTLA-4 with CD80/CD86 can inhibit T-cell activation.
-
3. Herpesvirus entry mediator (HVEM) & B- and T-lymphocyte attenuator (BTLA)/CD160
HVEM, also known as tumor necrosis factor receptor superfamily member 14 (TNFRSF14), is a human cell surface receptor of the TNF-receptor superfamily. BTLA is an Ig superfamily co-inhibitory receptor with structural and functional similarities to PD-1 and CTLA-4. The regulation of cell death through BTLA signaling is a key determinant of the inflammatory response. The ligation of HVEM and BTLA can activate inhibitory signaling in T-cells and could play a role in evading host anti-tumor immunity. CD160 is an identified ligand for HVEM.
The binding of BTLA or CD160 to HVEM delivers a co-inhibitory signal. Therapies blockade of HVEM &BTLA /CD160 are being developed to enhance immune responses and vaccination.
-
4. Lymphocyte-activation gene 3 (LAG-3)
LAG-3 plays important roles in the negative regulation of T-cell response, which makes it a potential target for immune modulation. Blockade of LAG-3 can enhance anti-tumor T-cell responses. Co-blockade of the LAG-3 and PD-1 pathways is more effective for anti-tumor immunity than blocking either molecule alone.
-
5. T-cell immunoglobulin and ITIM domain (TIGIT) & CD155
TIGIT is an important inhibitory molecule within the PVR/nectin family that is associated with human cancers and T-cell exhaustion phenotypes. Studies using knockout mice demonstrate that this pathway is important in optimal NK effector cell function (activation and/or tolerance). The ligand for TIGIT is CD155 (also called poliovirus receptor) that is also a ligand for CD226, an immune costimulatory receptor involved in antiviral and antitumor responses. TIGIT negatively modulates NK cell killing and T-cell activation via two mechanisms: competing with CD226 for binding to CD155 on adjacent T-cells, and directly preventing CD226 homodimerization and signaling. Therefore, the TIGIT & CD155 regulatory pathway is emerging as a new target for immune checkpoint blockade strategies.
-
6. T-cell immunoglobulin and mucin domain 3 (TIM-3) & Galectin-9 / Carcinoembryonic antigen-related cell adhesion molecule 1(CEACAM1)
TIM3, a member of the recently discovered Tim superfamily, is a negative regulator of TH1 immunity. TIM-3 is highly expressed on mature human NK cells and is variably expressed on immature NK cells. TIM-3 marks NK cells with greater effector function, including cytokine production and cytotoxicity. Therefore, TIM-3 is a great potential target alone or in combination with current PD-1 and CTLA-4-based immunotherapy of cancer. TIM-3 & Galectin-9 pathway is important for the regulation of dNK cell function, which is beneficial for the maintenance of a normal pregnancy.
CEACAM1, also known as CD66a, is a human glycoprotein, and a member of the carcinoembryonic antigen (CEA) gene family. CEACAM1 is an extensively studied cell surface molecule with established functions in multiple cancer types, as well as in various compartments of the immune system. Due to its important roles as a recently appreciated immune checkpoint inhibitor and tumor marker, CEACAM1 is an attractive target for cancer immunotherapy.
-
7. Indoleamine 2,3-dioxygenase 1 (IDO1)
IDO1 is an enzyme produced in macrophages within the tumor microenvironment that acts on tryptophan metabolism. IDO1 decreases proliferation of T-cells and increases neovascularization by countering interferon gamma. It is an immunosuppressive molecule that has been under investigation for therapeutic intervention.
-
8. V-domain Ig suppressor of T-cell activation (VISTA)
VISTA, also known as PD1 homolog (PD1H), C10orf54 or Dies1, belongs to the B7 family. VISTA encodes for a type I membrane protein and is expressed predominantly on hematopoietic cells, e.g., myeloid, granulocytic and T-cells. There is an ongoing cancer immunotherapy clinical trial for monoclonal antibodies targeting VISTA in advanced cancer.
-
9. Signal regulatory proteins alpha (SIRPα) & CD47
Signal regulatory proteins (SIRP) are a multigene family of immune receptors encoded by a cluster of genes. SIRPα, also called CD172a, is one of the five members (SIRPα, SIRPβ1, SIRPγ, SIRPβ2, and SIRPδ) and is the best-conserved member. CD47 is highly expressed in many different types of cancer, and it transduces inhibitory signals through SIRPα on macrophages and other myeloid cells. The NH2-terminal CD47-ligand binding V-domain of SIRPα can bind to CD47 to inhibitory signaling capacity. The SIRPα & CD47 immune checkpoint pathway is a critical regulator of myeloid cell activation and serves a broader role as a myeloid-specific immune checkpoint. A number of therapeutics that target the SIRPα & CD47 are under preclinical and clinical investigation.
-
10. 2B4 & CD48
2B4, also known as CD244, is a member of the CD2 subset of the Ig superfamily. 2B4 is mainly expressed on innate immune cells, including NK cells, and on subsets of T-cells. The 2B4 molecule interacts with CD48, which is widely expressed on hemopoietic cells. CD48, a glycosyl-phosphatidyl-inositol (GPI)-anchored cell surface protein, is a proposed physiological ligand for the activating receptor 2B4. Upon binding to CD48 or when cross-linked with antibodies, 2B4 initiates increasing cytotoxicity and cytokine secretion.
-
11. B7-H3
B7-H3, also known as CD276, is an important immune checkpoint member of the B7 and CD28 families. Induced on antigen-presenting cells, B7-H3 plays an important role in the inhibition of T-cell function. B7-H3 often correlates with both negative prognosis and poor clinical outcome in patients. B7-H3 is highly overexpressed on a wide range of human solid cancers. Based on the clinical success of inhibitory immune checkpoint blockade (CTLA-4, PD-1, and PD-L1), monoclonal antibodies against B7-H3 become a promising therapeutic strategy worthy of development.
-
12. V-set domain containing T-cell activation inhibitor 1 (VTCN1)
VTCN1, commonly known as B7-H4, has been identified as a potential therapeutic target for the treatment of cancer. Since B7-H4 is expressed on tumor cells and tumor-associated macrophages in various cancer types, therapeutic blockade of B7-H4 could favorably alter the tumor microenvironment allowing for antigen-specific clearance of of tumor cells.
Creative Biolabs has developed a cutting-edge SuPrecision™ platform for whole exome sequencing (WES), which is focused on coding regions of the genome. Based on this cutting-edge platform, we have accumulated extensive in performing whole exome sequencing (WES) and TMB analysis for response to checkpoint inhibitor immunotherapy. Through our one-stop solution system, we can offer high-quality service of TMB analysis for response to checkpoint inhibitor immunotherapy at the most competitive price.
Please contact us for more information and a detailed quote.
Reference
- Greisen, Stinne R., Maithri Aspari, and Bent Deleuran. "Co-inhibitory molecules–their role in health and autoimmunity; highlighted by immune related adverse events." Frontiers in immunology 13 (2022): 883733. Distributed under open access license CC BY 4.0, without modification.
Resources
Infographics
Podcast
- SuPrecision™ Sequencing Applications
- Co-stimulatory Immune Checkpoint Pathways
- Podcasts
- Download Center