All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Melanoma-associated antigen A4, also named MAGE-A4, is a potential tumor-associated antigen overexpressed on various solid tumors. The low affinity of natural TCR binding on MAGE-A4 epitope inspired scientists to engineer the T cell with exogenous TCR for antigen recognition and induce enhanced antitumor activities. In this article, a specific, high-affinity TCR-T cell product is produced from patient-derived T cells by lentivirus particle transfection of degraded T cells. This article reports the results of the phase 1 trial of this autologous TCR cell therapy among HLA-A2+ MAGE-A4+ cancer patients.
Melanoma-associated antigen A4, also named MAGE-A4, is a potential tumor-associated antigen overexpressed on various solid tumors, such as sarcoma, head and neck cancer, non-small-cell lung cancer (NSCLC), ovarian cancer, melanoma, and urothelial cancer. Commonly, MAGE-A4 is processed into small peptides and presented on the cell surface with HLA molecules to induce T cell immunity in vivo.
The low affinity of natural TCR binding on MAGE-A4 epitope inspired scientists to engineer the T cell with exogenous TCR for antigen recognition and induce enhanced antitumor activities. In this article, a specific, high-affinity TCR-T cell product is produced from patient-derived T cells by lentivirus particle transfection of degraded T cells. The TCR is designed to target a specific epitope of MAGE-A4, GVYDGREHTV, which is presented by most HLA-A*02 alleles.
This article reports the results of the phase 1 trial of this autologous TCR cell therapy. HLA-A2+ patients examined with MAGE-A4-expression are enrolled, and 38 patients are treated with the candidate TCR-T cells with doses ranging from 0.08 × 109 to 10 × 109 cells.
The safety and toxicity of the therapy are evaluated based on diverse adverse events, including multiple immune and blood cells count decreases, CRS, mental state, physiological reactions, and so on. Grade ≥3 hematologic toxicities are monitored in all patients; most patients (82%) experienced low-grade cytokine release syndrome; 5% of patients suffered Grade 1 neurotoxicity syndrome, which is controllable.
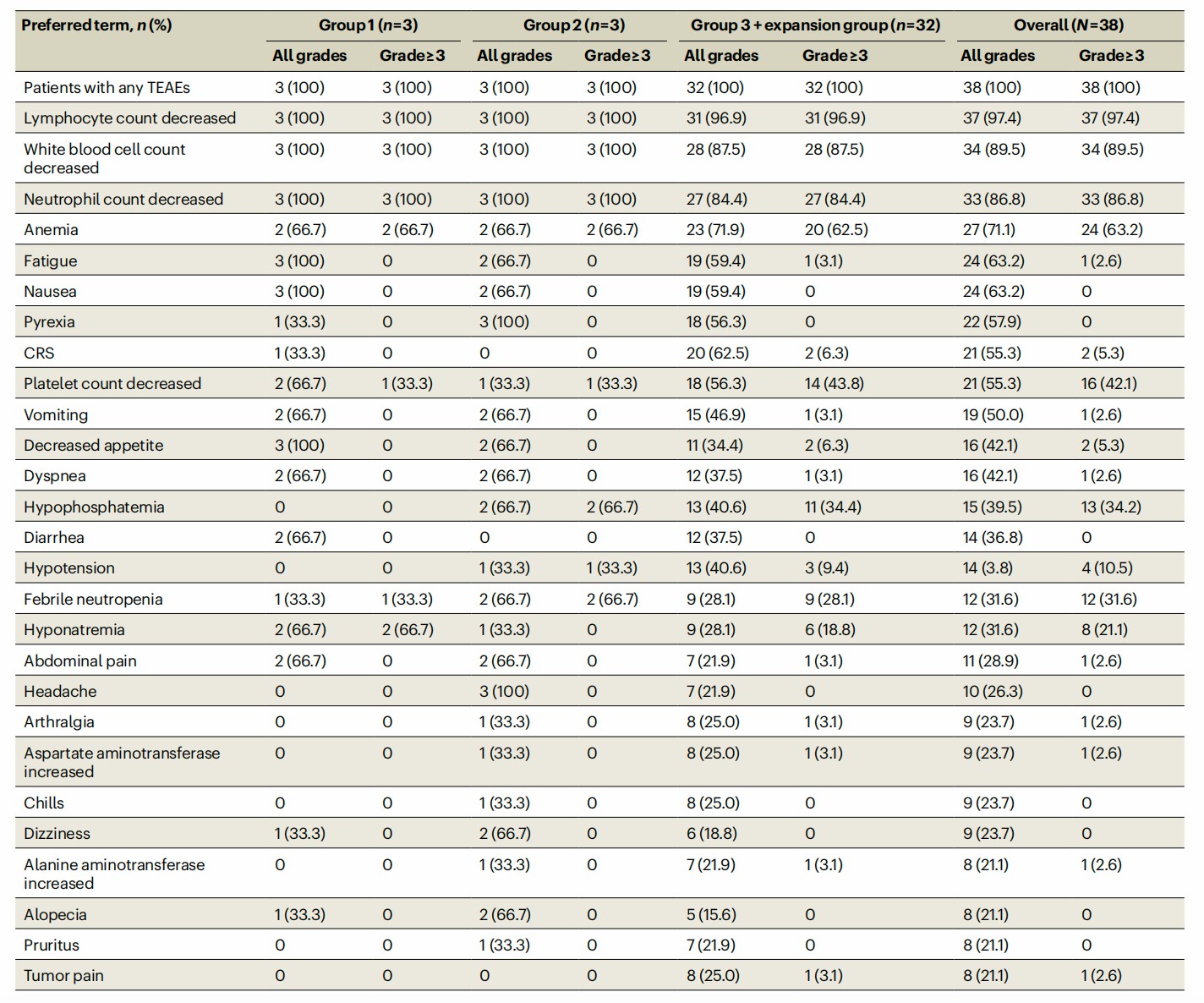 Fig.1 Clinical record of adverse events during Anti-MAGE-A4 TCR-T treatment. (Hong, et al., 2023)
Fig.1 Clinical record of adverse events during Anti-MAGE-A4 TCR-T treatment. (Hong, et al., 2023)
The antitumor activity of anti-MAGE-A4 TCR-T therapy varies with cell dose. The longest diameter (SLD) of specific lesions is measured before and after treatment and is used to determine the disease control rate. Data showed the overall partial regression rate is 24%, and the response rate is higher in synovial sarcoma (SS) patients and minimal in other cancer types, like NSCLC and ovarian cancer. The median response time of the therapy is 25.6 weeks and 28.1 weeks for all patients and SS patients.
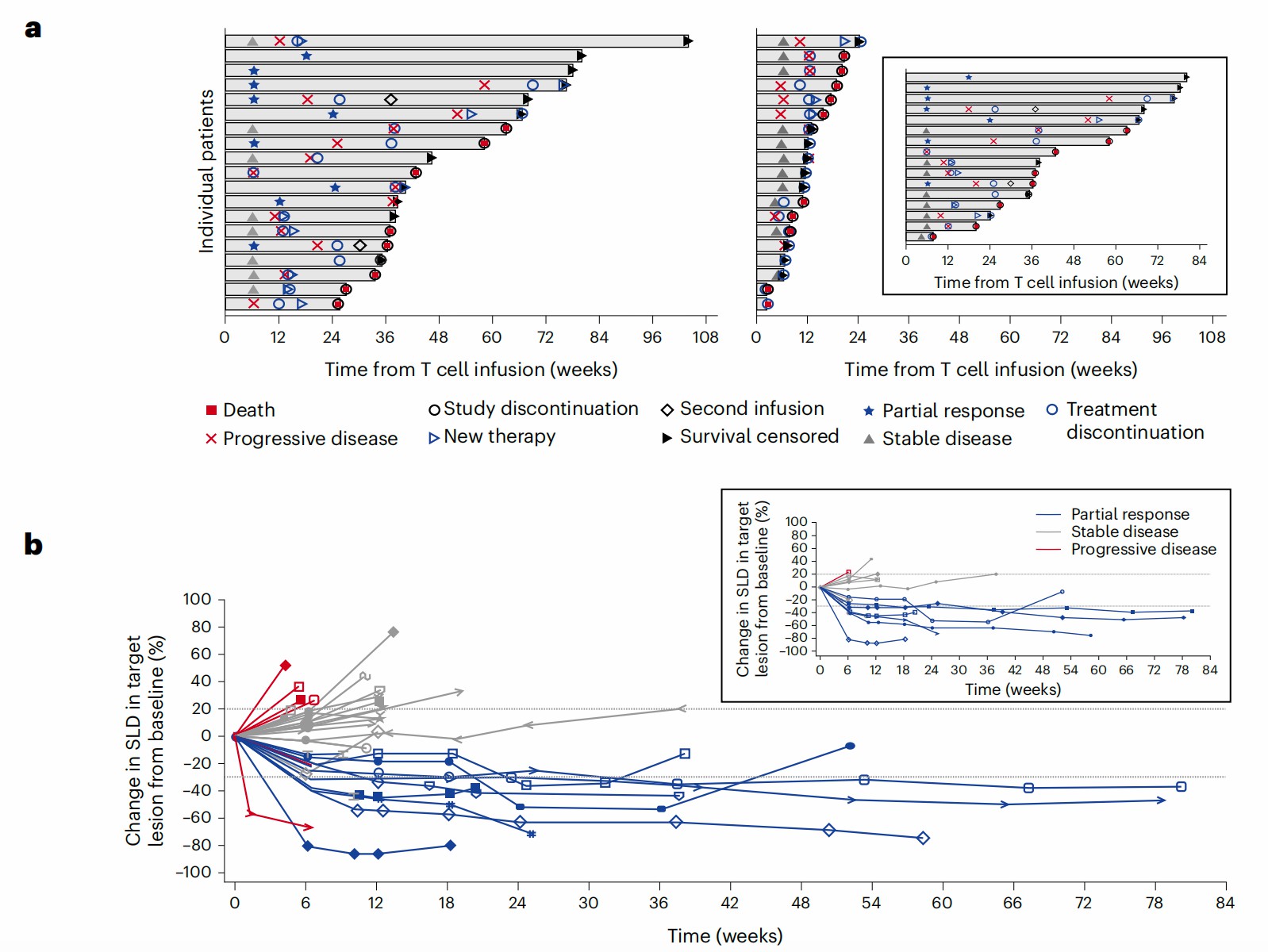 Fig.2 Clinical results of SS patients' responses to MAGE-A4 targeted TCR-T therapy.1
Fig.2 Clinical results of SS patients' responses to MAGE-A4 targeted TCR-T therapy.1
The adoptive TCR-T cells that exist in peripheral blood and intratumor are isolated and studied for immunophenotype, cytotoxicity, cytokine release analysis, and tumor microenvironment alterations are characterized. Exploratory experiments demonstrated that the engineered T cells could migrate into tumor tissues, perform interferon-γ-dependent cytotoxicity, and induce system immune responses at the same time.
According to these clinical results, this anti-MAGE-A4 TCR-T cell therapy has early and durable antitumor function toward HLA-A2 SS patients and controllable risk.
With cutting-edge technologies and skilled scientists, Creative Biolabs offers tailored TCR-T development services based on our powerful immunotherapy platform. Our senior experts and experienced technicians allow us to design innovative strategies to fulfill your project with reproducible data and high efficiency.
One-Stop TCR-T Therapy Development
Creative Biolabs is a leading biotechnology company that has enriched experience in immunotherapy exploration and translation. We provide a validated and mature working procedure for TCR-T cell construction, in vitro and in vivo assessment, and production to meet strict regulations and standards.
Creative Biolabs has rich experience in T cell engineering with functional CARs and TCRs, and we also provide various immune cells, engineering tools, and validation kits for clients to help them study novel immunotherapies.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION