All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Dedicated to excellence, Creative Biolabs is a CRO solution company run by a group of knowledgeable scientists and specialists with extensive experience in cell therapy R&D. We offer unparalleled products and services to clients in both the academic and industrial sectors worldwide, with the goal of facilitating breakthrough scientific discoveries and advancing preclinical and clinical research.
CD171 is a glycoprotein target, also known as L1 cell adhesion molecule (L1CAM). It plays a key role in neuronal development and synapse formation and is widely recognized as a marker for specific tumors, such as neuroblastoma (NB). CD171 has a similar structure to other glycoproteins, with an extracellular structural domain, a transmembrane region, and an intracellular structural domain. It has an expression pattern that overlaps with other glycoproteins, such as CD56 and CD114. As such, it is often used in immunohistochemistry and flow cytometry to identify neurons and specific tumor cells. CD171 is a gene in the human genome with a size of approximately 37 kb, consisting of 27 exons and 26 introns. At the protein level, CD171 encodes a protein called neural cell adhesion molecule 1 (NCAM1), which contains about 839 amino acid residues. CD171 has typical immunoglobulin structural domains, including six Ig-like and five fibronectin type III-like structural domains. The sixth Ig-like domain differs from other Ig-like domains in that it contains an RGD sequence at the N-terminal end that binds to integrins in the extracellular matrix. CD171 plays a critical role in embryonic development and neurogenesis, being involved in neuronal migration, axon guidance, synapse formation, and other processes. Additonally, CD171 has been associated with the development and metastasis of various cancers.
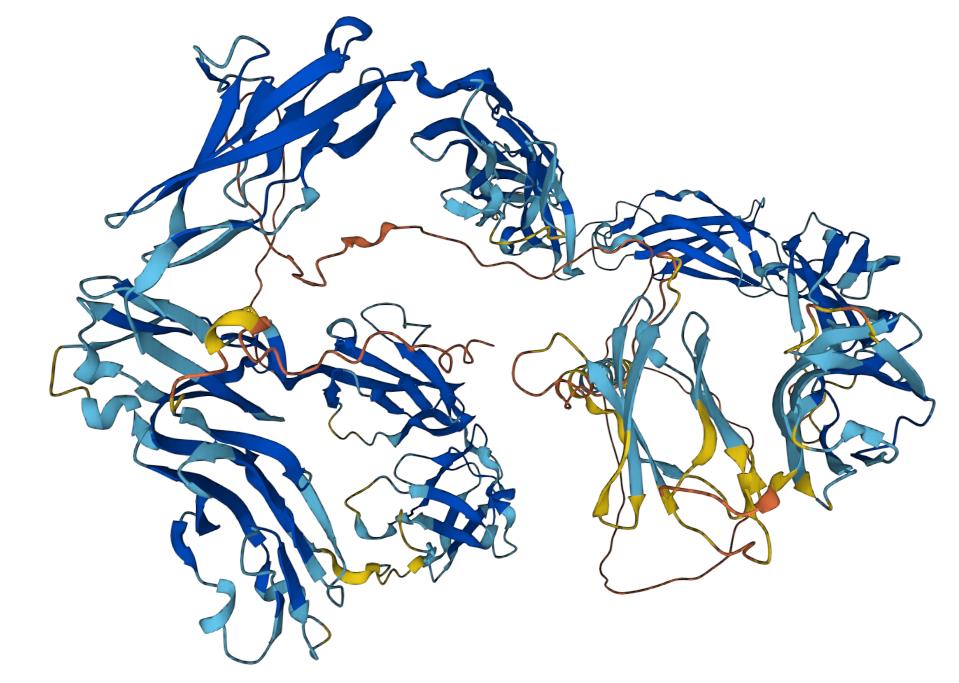 Fig.1 Structure of CD171
Fig.1 Structure of CD171
Recent studies have shown that CD171 activates the inositol phospholipid-3-kinase (PI3K)/protein kinase B (AKT) signaling pathway, thereby promoting cancer cell proliferation, invasion, and metastasis. For example, CD171 is highly expressed in NB and is associated with tumor grade, prognosis, and treatment response. A similar expression pattern is seen in other tumors such as melanoma, colorectal cancer, and breast cancer. CD171 interacts with PI3K through its intracellular structural domain, promoting PI3K activation and AKT phosphorylation. These events lead to the activation of downstream effectors such as mTORC1 and FOXO1/3a, which promote cancer cell proliferation, metabolism, and invasion. In addition, CD171 promotes cancer cell metastasis and invasion through activation of signaling pathways such as SRC family protein tyrosine kinases and Focal adhesion kinase (FAK). The Notch signaling pathway is conserved among species and plays a key role in embryonic development and tissue homeostasis. CD171 has been shown to interact with the Notch signaling pathway through its interaction with the Notch ligand Jagged1. CD171 promotes the Notch signaling pathway by enhancing Jagged1 expression and increasing NICD levels. This interaction between CD171 and Jagged1 is involved in multiple biological processes, including cancer progression and neuronal development.
In cancer, CD171-mediated activation of Notch signaling has been shown to promote tumor growth, invasion, and metastasis. For example, in ovarian cancer, CD171 expression is upregulated and promotes tumor growth and metastasis through the activation of Notch signaling. Similarly, in neuronal development, CD171 has been shown to promote axonal growth and guidance through interaction with Jagged1 and activation of Notch signaling. Mutations in CD171 are associated with several neurological disorders, including X-linked hydrocephalus and spastic paraplegia type 1, which are characterized by abnormal neuronal migration and axonal guidance. In addition to interacting with Jagged1, CD171 has been shown to interact with other Notch ligands, including Delta-like 4 (DLL4). CD171 promotes DLL4 expression and enhances the DLL4-mediated Notch signaling pathway, leading to downstream effects on angiogenesis and cancer progression. Additionally, CD171 has been associated with several neurological disorders, such as X-linked childhood movement disorders and brain dysplasia.
Chimeric antigen receptor (CAR) T cell therapy modifies T cells to express a CAR that recognizes a specific antigen. The CAR comprises an extracellular structural domain that identifies the target antigen, a transmembrane structural domain, and an intracellular structural domain that activates T-cell signaling pathways when the antigen is recognized. CD171 CAR-T-cell therapy modifies T cells to express a CD171-specific CAR, which targets and kills cancer cells that overexpress CD171. Several preclinical studies have demonstrated the effectiveness of CD171 CAR-T cell therapy in vivo and in vitro. These studies show that CD171 CAR-T cells can eliminate CD171-positive tumor cells, leading to tumor regression and prolonged survival in an ovarian cancer xenograft model. CD171 CAR-T cells have also shown specific cytotoxicity against CD171-positive pancreatic and colon cancer cells. CD171 CAR-T cell therapies are still in the early stages of development In clinical trials. Most of the phase I clinical trials are assessing the safety and efficacy of CD171 CAR-T cells in patients with relapsed or refractory cancers. This is because CD171 expression levels may vary between different types of cancer and different tumor subtypes. In addition, CD171 is also expressed in normal tissues. To address these challenges, future research directions for CD171 CAR-T cell therapy could focus on optimizing CAR design to improve specificity and reduce potential toxicity. For example, incorporating a safety switch in CARs could control the activity of CAR-T cells in the event of adverse reactions. Moreover, identifying other targets in combination with CD171 could enhance the efficacy of CAR-T cells and reduce the risk of tumor escape.
Table 1. Ongoing CD171-Targeted CAR Cell Therapy Clinical Trials
| NCT Number | Title | Status | Study Results | Conditions | Sponsor/Collaborators | Phases |
| NCT02311621 | Engineered Neuroblastoma Cellular Immunotherapy (ENCIT)-01 | Active, not recruiting | No Results Available | Neuroblastoma | Ganglioneuroblastoma | Seattle Children's Hospital|The Evan Foundation|Ben Towne Center for Childhood Cancer Research | Phase 1 |
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION