All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Lipid Nanoparticles (LNPs)-Mediated CAR mRNA Delivery
Unlike genetic transformation methods with plasmid or viral vectors, in vitro transcription (IVT)-mediated transient expression of CAR mRNA is safer in cancer immunotherapy. Since the naked mRNA has a short half-life, drug delivery systems such as lipid nanoparticles (LNPs) are necessary to protect mRNA from degradation and enhance cellular uptake. Creative Biolabs has established a CAR IVT platform to support IVT mRNA & LNP manufacturing. We provide full technical support from project design, sequence optimization, and synthesis, to LNP formulation services. Our scientists will encapsulate mRNA with lipids based on specific requirements.
The advantage of the LNP delivery system:
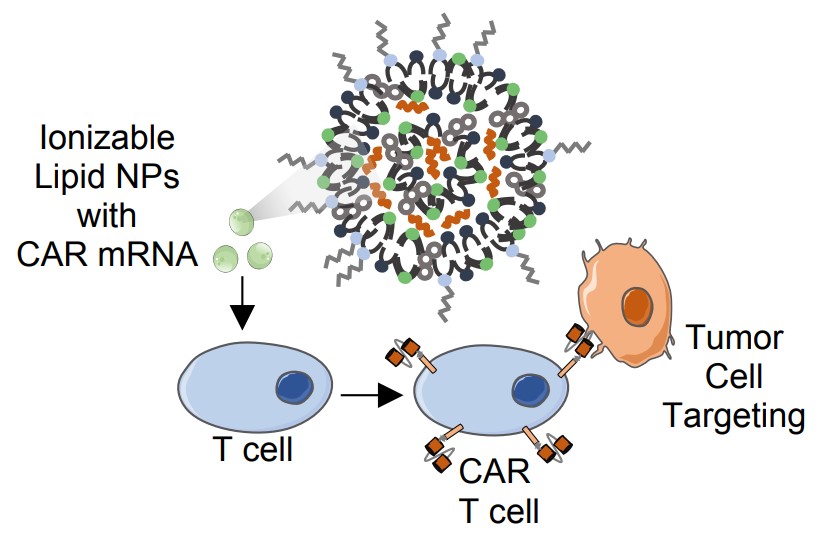 Fig.1 LNPs-mediated CAR mRNA delivery to human T cells.1
Fig.1 LNPs-mediated CAR mRNA delivery to human T cells.1
CAR IVT LNP Products and Services
Creative Biolabs proudly offers CAR IVT LNP products and services for mRNA-based CAR therapy research and development. We have a purpose-built LNP delivery system for effective encapsulation of mRNA. Moreover, we have optimized our manufacturing strategy to maximize yields and ensure the efficacy of encapsulation. Our integrated CAR IVT LNP manufacturing workflow streamlines production from gene synthesis to LNP packaging. Our CAR IVT LNP products help save time and streamline your efforts.
For any specific requirements or technological support, please contact us.
Quality Control
To ensure that CAR IVT LNP is properly synthesized and loaded with the intended mRNA, Creative Biolabs will perform standard and customized quality control assays, including:
Manufacturing Workflow
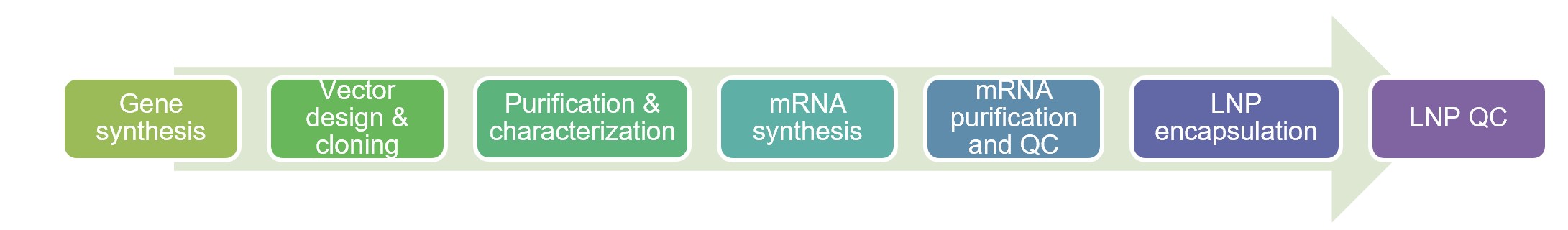 Fig.2 Workflow of CAR IVT LNP manufacture.
Fig.2 Workflow of CAR IVT LNP manufacture.
Reference
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION