All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Cancer immunotherapy with immune checkpoint inhibitors has recently emerged as a powerful tool for the treatment of diverse advanced malignancies. Although the pharmacodynamics of these immune modulators are complex, recent studies strongly support the notion that altered peptide ligands presented on tumor cells representing neoantigens may play an essential role in tumor rejection. Neoantigens are derived from single nucleotide variants (SNVs) and small insertions and deletions (indels). Identification of tumor neoantigens has been facilitated by the development of high-throughput sequencing data. Equipped with a team of seasoned scientists with an advanced immune monitoring platform designed specifically to meet very challenging requirements in biomarker development, Creative Biolabs offers fast, reliable support for any phase of neoantigen biomarker development.
Neoantigens arise from any genomic mutation altering protein sequence, including non-synonymous mutations, retained introns, post-translational modification (PTM) that alters amino acid, gene fusions, and frameshift indels. As the genomic variations are specific to cancer cells and are not present in the germline, they are not subject to central and peripheral tolerance. Therefore, these variations are also known as tumor mutated specific antigens (TMSAs), which allow tumors sufficiently distinct from normal tissue to activate the immune system and induce an efficient anti-tumor response. The developments of innovative next-generation sequencing (NGS) along with advances in bioinformatics have enabled systemic analysis of the mutation load of the tumor as well as identification of each type of the potentially immunogenic neoantigens, except for PTM, which relies on technologies such as mass spectrometry (MS). In recent years, although the number of identified immunogenic neoantigens has substantially increased, with respect to neoantigen biomarker development for cancer immunotherapy, a greater number of neoantigens is needed to be predicted and verified.
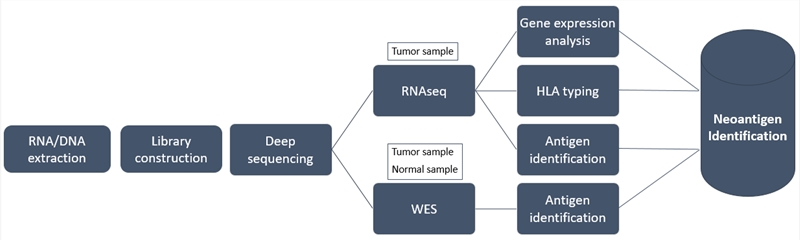
With industry-leading expertise and state-of-the-art equipment, Creative Biolabs has pioneered the discovery of neoantigen biomarkers. The prediction of neoantigens relies on the bioinformatics analysis of genomic data and requires knowledge of HLA type, tumor mRNA expression, germline DNA and tumor DNA. Combining whole-exome sequencing (WES) and transcriptome sequencing with our optimized neoantigen prediction algorithm, scientists at Creative Biolabs provide a comprehensive analysis of candidate neoantigens in a tumor and matched normal sample.
Creative Biolabs is well equipped and versed in candidate neoantigen identification. We are glad to serve our global clients with professionalism and expertise in neoantigen biomarker discovery. For more information on our neoantigen identification service, please feel free to contact us and further discuss with our scientists.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION