Creative Biolabs provides a one-stop service for recombinant factor C assays.
Creative Biolabs provides the advanced recombinant Factor C (rFC) method as an improved alternative to the classic LAL assay. Unlike LAL, which uses horseshoe crab blood and can produce false positives due to G-factor interference, our rFC approach has superior specificity and accuracy.
With improved sensitivity, a broader detection range, and consistent performance across batches, our rFC service provides reliable results you can trust. Additionally, it supports sustainability by eliminating the need for animal-based materials. Recognized by the regulatory authorities, our rFC solution is ideal for high-throughput testing. Choose Creative Biolabs for precise, eco-friendly endotoxin detection.
A possible validation timeline is outlined below. The test with the rFC method is performed in the step of validation of the alternative method.
How does rFC differ from the traditional Limulus Amebocyte Lysate (LAL) assay?
Unlike the LAL assay, which relies on horseshoe crab blood cells, rFC is entirely animal-free, supporting sustainability and reducing the impact on endangered horseshoe crab populations. The rFC method also avoids interference from the G-factor, which can cause false positives in the LAL assay. This increases the specificity and reliability of rFC, providing more consistent and accurate results across various applications.
What equipment is required for rFC testing?
Performing rFC testing requires a fluorescence microplate reader capable of detecting at an excitation wavelength of 380 nm and an emission wavelength of 440 nm. The assay is usually conducted at 37°C ± 1°C to maintain optimal reaction conditions. The reader must be compatible with 96- or 384-well plates for high-throughput applications.
Is the rFC method internationally recognized?
Yes, the rFC method has gained significant recognition worldwide. It has been approved to be an alternative to
the LAL assay for endotoxin testing in pharmaceutical, and it has been included in the European Pharmacopoeia since 2015. Its acceptance by regulatory bodies demonstrates its reliability and alignment with international quality standards.
Can rFC be used with all sample types?
Yes, the rFC method works well with a wide variety of sample types, including those containing substances like β-glucan, which can interfere with the LAL assay. Its high specificity ensures accurate results, even for complex samples such as biological drugs, medical devices, and injectable solutions. If you are not sure about your sample type, please feel free to send us an inquiry now!
What is the future outlook for rFC?
The rFC method is expected to become the standard for endotoxin testing, especially as regulatory agencies continue to support animal-free alternatives. As production technology advances and rFC becomes more accessible, it is likely to fully replace
the LAL assay. Its combination of high accuracy, sustainability, and compliance makes it an ideal solution for future pharmaceutical testing needs.


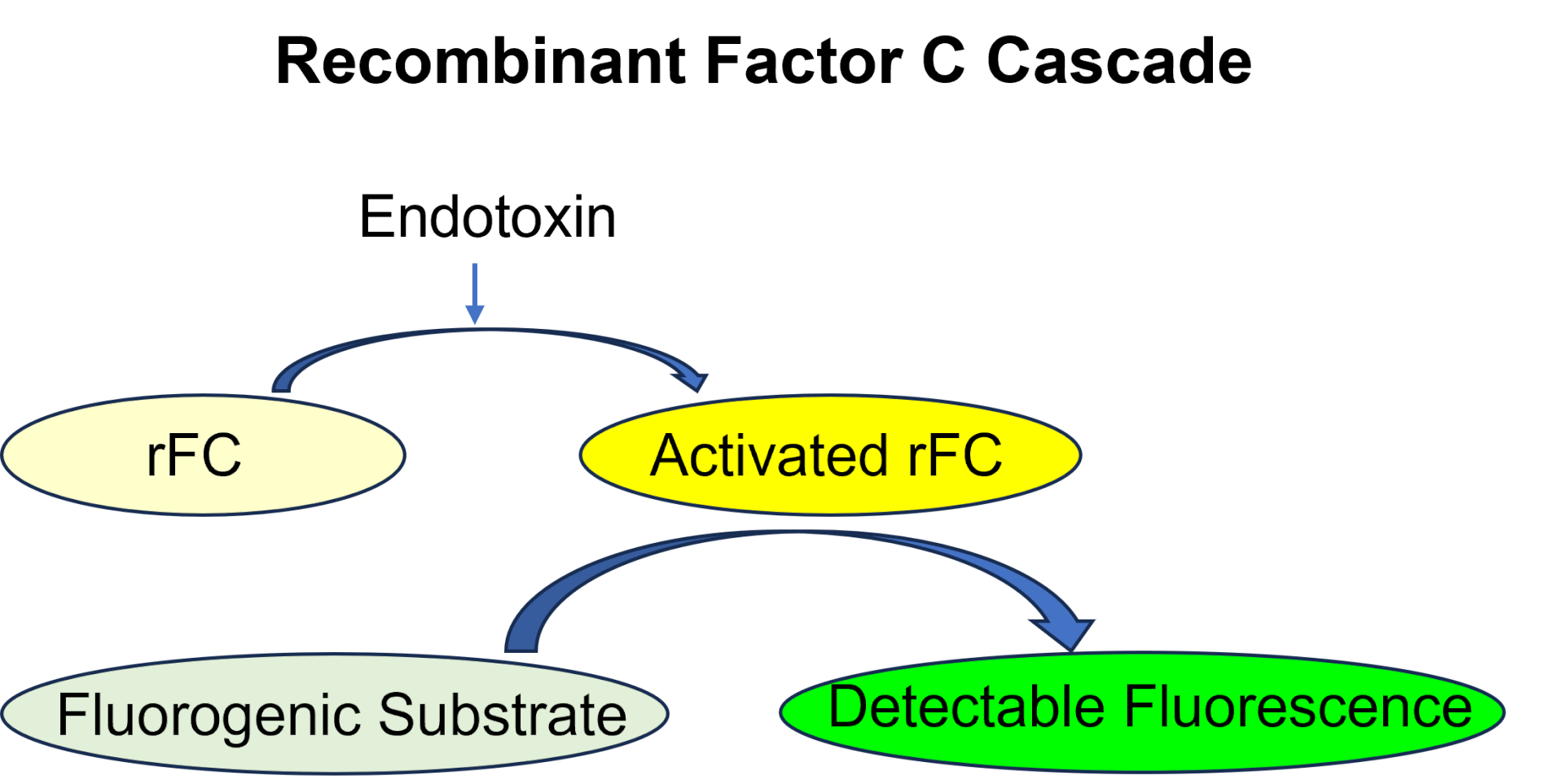 Fig.1 The Novel Single-Step Activation of Recombinant Factor C Assay.
Fig.1 The Novel Single-Step Activation of Recombinant Factor C Assay.
 Fig.2 Validation Timeline.
Fig.2 Validation Timeline.
 Download our brochure
Download our brochure