Variable Region Sequencing & Cloning Service
Background Workflow Publication Why Choose Us FAQs Customer Review Related Services Contact Us
Region Sequencing and Cloning for Antibody Engineering and Therapeutic Development
As a critical step of antibody molecular biology services, antibody variable region (VH and VL) sequencing and cloning are very important for antibody construction. Creative Biolabs is committed to providing a one-stop solution for superior therapeutic antibody discovery and development, and we offer this key first step-variable region sequencing and cloning service.
Antibody variable region sequencing from hybridomas or clonal B cells is a critical first step in a broad range of applications, such as the production of recombinant antibodies in different formats, antibody humanization and affinity maturation, antibody engineering, etc. At Creative Biolabs, we offer variable region sequencing services, including heavy and light chains from both hybridoma cell lines and clonal primary B cells derived from a wide range of species. Following the antibody variable region sequencing service, the variable regions can be reformatted into various types and transiently be produced in non-mammalian or mammalian cells. Once sequenced, we offer cloning services to deliver both sequence results and clone constructs to our clients.
Comprehensive Workflow for Variable Region Sequencing and Cloning
Our variable region sequencing and cloning workflow at Creative Biolabs is meticulously designed for clarity, efficiency, and accuracy, ensuring comprehensive and reliable results for your project. Each stage is optimized to provide maximum insight and control over your antibody development.
Required Starting Materials:
-
Hybridoma cells (frozen or live): These cells are the primary source of antibody-producing genetic material.
-
B cell samples: For projects requiring immune repertoire analysis or direct B cell cloning.
-
Antigen information: This can include the antigen's sequence, structural data, or purified protein, which aids in understanding antibody-antigen interactions and validating specificity.
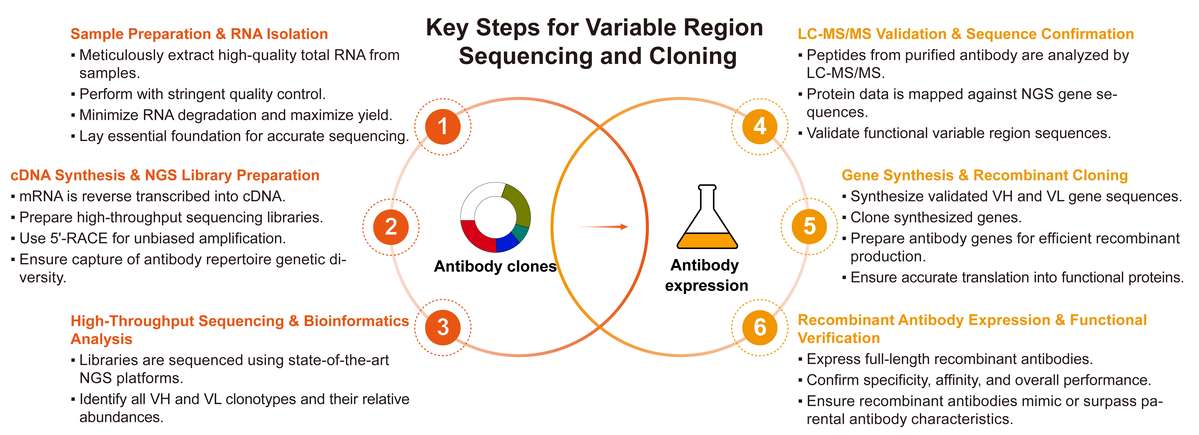
Final Deliverables:
-
Validated VH and VL nucleotide and amino acid sequences: The precise genetic and protein sequences of your antibody's variable regions.
-
A comprehensive bioinformatics report: This report details the repertoire diversity, clonotype abundance, and any identified variants.
-
Expression vectors containing your cloned antibody genes: Ready for your downstream expression.
-
Purified recombinant antibody (upon request): Accompanied by detailed functional characterization data, including binding kinetics and specificity.
Publication
This publication addresses the challenges of obtaining accurate variable region (VR) sequences from hybridoma-derived monoclonal antibodies (mAbs) due to their polyploidy. The study introduces a novel and efficient approach combining LC-MS/MS with next-generation sequencing (NGS) and specific PCR to characterize the VR sequences of an anti-thiacloprid mAb. The accuracy of these identified VR sequences was validated by expressing a functional full-length recombinant antibody (rAb) in mammalian cells, which showed similar performance to the parental mAb. Furthermore, through molecular docking and single-site-directed alanine mutagenesis, the research successfully identified key amino acid residues in the complementarity-determining regions (CDRs) crucial for thiacloprid-specific recognition, which is vital for improving antibody properties. This methodology provides a reliable way to determine recognition sites in hapten-specific mAbs, enhancing immunoassay development for environmental and agricultural contaminants like thiacloprid.
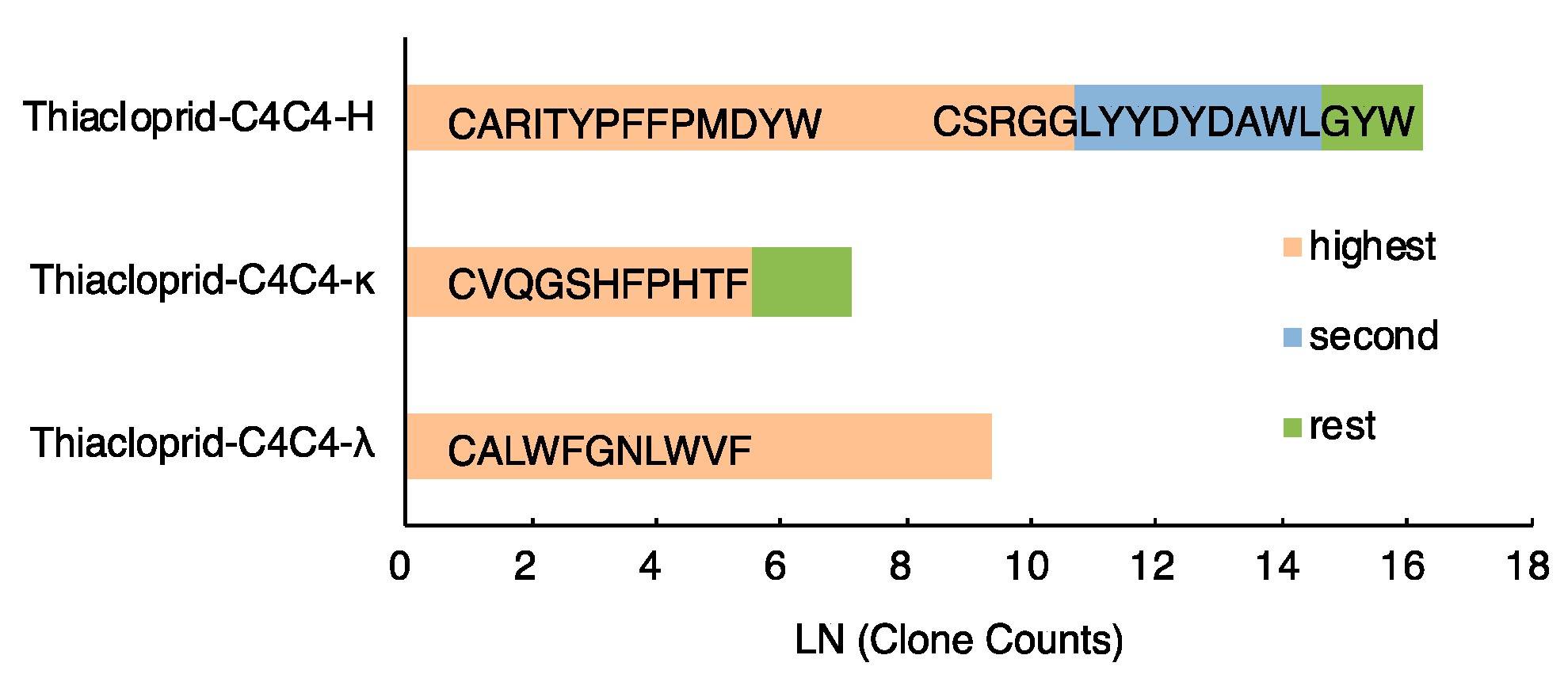 Fig.1 Clonal analysis of heavy chain variable regions.1
Fig.1 Clonal analysis of heavy chain variable regions.1
Why Choose Us?
Creative Biolabs is a leader in antibody engineering, offering unique advantages through its Variable Region Sequencing and Cloning service:
-
Integrated validation for unparalleled accuracy: Combines NGS and LC-MS/MS for highly accurate functional antibody sequence identification, overcoming hybridoma complexities and reducing the risk of non-functional sequences.
-
Mammalian expression expertise for optimal functionality: Prioritizes full-length IgG expression in mammalian systems to ensure proper folding, glycosylation, and assembly, resulting in recombinant antibodies matching native activity.
-
Rational design capabilities for enhanced antibody properties: Utilizes advanced molecular docking and site-directed mutagenesis for deep insights into antigen binding, enabling targeted affinity maturation and rational design of improved antibodies.
-
End-to-end solution for streamlined development: Offers a seamless, integrated workflow from initial sequence identification to validated recombinant antibody production, saving time and resources.
Experience the Creative Biolabs Advantage - Get a Quote Today and Propel Your Antibody Research Forward!
FAQs
Q1: Why is it so difficult to get the correct variable region sequences from my hybridoma using standard PCR, and how does Creative Biolabs overcome this?
A1: Hybridomas' polyploid nature can lead to incorrect sequencing. Creative Biolabs overcomes this with an integrated NGS and LC-MS/MS approach. This precisely identifies and validates functional antibody sequences at the protein level, ensuring unparalleled accuracy.
Q2: How does Creative Biolabs ensure the recombinant antibody will perform like my original hybridoma-derived antibody?
A2: We prioritize expressing full-length recombinant IgG in mammalian cells (e.g., HEK293). This ensures proper folding, glycosylation, modifications, and assembly, critical for maintaining native binding and activity. This meticulous approach guarantees the recombinant antibody closely mimics your parental mAb's performance.
Q3: My project involves a large number of hybridomas or extensive immune repertoire analysis. Is Creative Biolabs' high-throughput approach scalable for my needs?
A3: Yes, our platform is built for scalability and high throughput. Our advanced sequencing efficiently analyzes numerous samples, ideal for large hybridoma projects or extensive immune repertoire profiling. Creative Biolabs is well-equipped to effectively meet your varying research and development demands.
Customer Review
-
Unmatched Accuracy!
Using Creative Biolabs' service in our research has significantly improved our ability to confidently select antigen-specific clones from our hybridomas. The LC-MS/MS validation step is a game-changer, eliminating the guesswork we faced with PCR-only methods. This precision has accelerated our lead optimization efforts. - Dr. A***n
-
Exceptional Functional Antibodies!
Creative Biolabs' focus on full-length IgG expression in mammalian systems delivered recombinant antibodies with binding characteristics virtually identical to our parental hybridoma-derived mAbs. This was crucial for our in vivo studies, avoiding issues we previously encountered with bacterial expression systems. Their service truly streamlined our preclinical development. - Prof. S***a Khan
Related Services
To further support your antibody development journey, Creative Biolabs offers a comprehensive suite of complementary services designed to accelerate your research and development efforts and maximize the potential of your antibodies.
Antibody Humanization Service
Recombinant antibodies show clinical success, but murine mAbs have immunogenicity issues. Humanized antibodies reduce this, making them promising alternatives. Creative Biolabs offers customized antibody humanization services, utilizing various methods and toolkits.
Learn More →
Antibody Reformatting and Production
Creative Biolabs provides cutting-edge technologies and services for therapeutic antibody discovery. We offer antibody reformatting and production services to facilitate early-stage development.
Learn More →
Contact Us
To meet your desired objectives, Creative Biolabs is dedicated to offering high-quality and efficient sequencing and cloning services to best tailor your projects. In addition, we also provide a full spectrum of functional assays to evaluate the antibody characteristics and functions. To get more information, please feel free to contact us.
Reference
-
Liu, Pengyan, et al. "Characterization of variable region genes and discovery of key recognition sites in the complementarity determining regions of the anti-thiacloprid monoclonal antibody." International Journal of Molecular Sciences 21.18 (2020): 6857. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3390/ijms21186857



 Fig.1 Clonal analysis of heavy chain variable regions.1
Fig.1 Clonal analysis of heavy chain variable regions.1
