In degenerative retinal diseases, particular cell types die. These include retinal ganglion cells (RGCs), retinal photoreceptors (PRs) and retinal pigment epithelium (RPE) cells, and they do not appreciably regenerate to restore lost function. Stem cells are an attractive source of cell therapy and stem cell therapy has been effective in preclinical models of various retinal degenerative diseases. Progenitor cells are similar to stem cells but their ability to self-renew or differentiate into multiple cell types is more limited. The mechanism of action (MOA) of retinal progenitor engraftment is believed to work through two main modes:
1. Retinal stem cells or progenitors differentiate and replace the diseased retinal PRs (light-sensing cells) and RPE cells and repair the damaged retinal tissue.
2. The transplanted cells release exosomes and soluble factors such as neurotrophic factors, cytokines, chemokines and growth factors, which act in a paracrine or endocrine manner and facilitate retinal tissue healing.
Cardiac progenitor cells (CPCs) show great potential as a cell resource for restoring cardiac function in patients affected by heart disease. CPCs generate cells of the three cardiac lineages: cardiomyocytes, smooth muscle cells and endothelial cells. The various CPCs reported to date are described below and their specific features are summarized in Table 1.
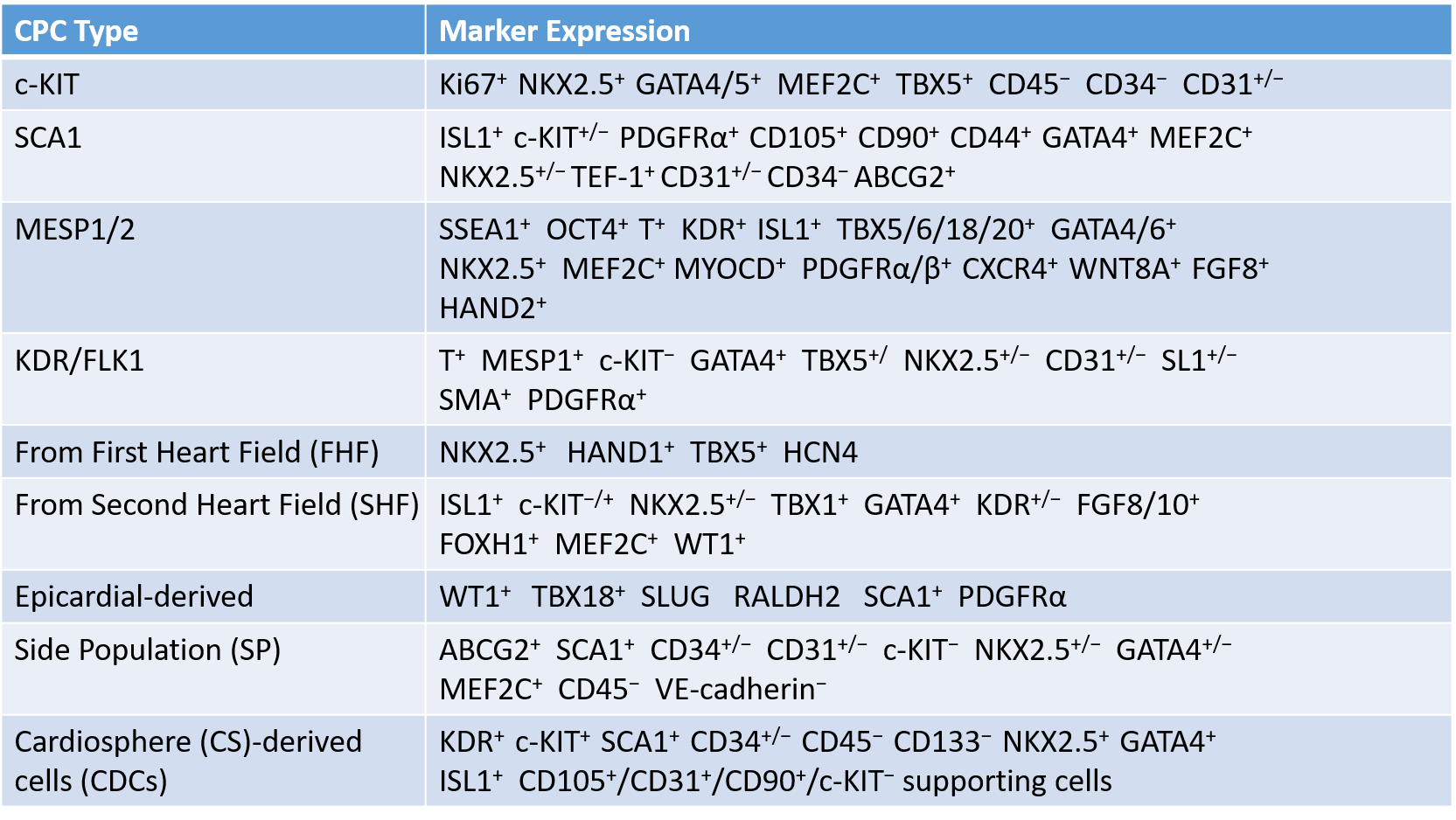
Table.1 Types of CPCs identified in the heart tissue.1
Expanded CPCs combined with small molecules and/or tissue engineering can be therapeutically transplanted into the damaged hearts of patients, such as those suffering from ischemic cardiomyopathy. Transplanted CPCs can be engrafted directly into the damaged host cardiac tissue and differentiated into mature cardiomyocytes as well as vascular cells. There has now been a multitude of clinical trials that have employed stem cell technologies for patients with ischemic cardiomyopathies, whose findings support the use of stem cell therapies in the heart to be safe.
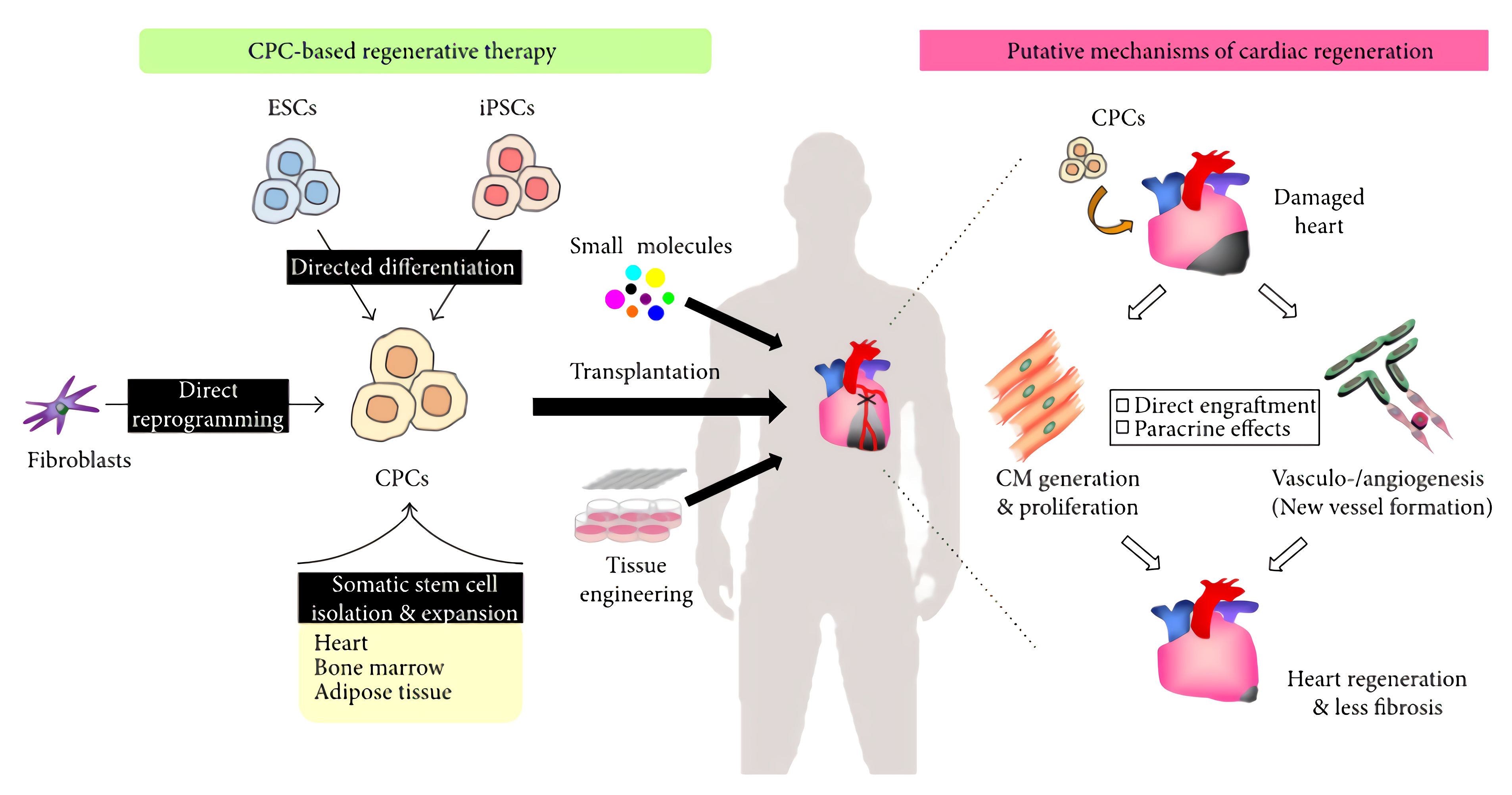 Fig.1 CPC-based regenerative therapy for heart disease.2
Fig.1 CPC-based regenerative therapy for heart disease.2
Hematopoietic stem and progenitor cells (HSPCs) can be generated from iPSCs. For a long time, myeloid differentiation bias has been considered a big barrier for deriving functional HSPCs from human iPSCs. Scientists showed that MLL-AF4-induced HSPCs (iHSPCs) were able to reestablish both myelopoiesis and lymphopoiesis without lineage bias in the recipient mice. By parallel comparison of iHSPCs with MLL-AF4-induced primary HSPCs, they also found that iHSPCs were more prone to leukemic transformation during the long-term engraftment period, providing a necessary caveat of using iHSPCs in the actual therapies.
The transplantation of neural stem/progenitor cells is regarded as a promising approach to the treatment of a range of central nervous system (CNS) disorders, such as amyotrophic lateral sclerosis (ALS), Parkinson's disease (PD), spinal cord injury, and brain infarction. iPSCs and numerous cell types derived from them have great potential in the field of CNS regeneration whether through direct cell replacement or creation of a pro-regenerative environment. Human iPSC-derived neural progenitor cells (NPCs) and neural crest stem cells (NCSCs) not only present a potential possibility for targeted cell replacement, but they also provide a tool for clinical application in cellular therapy.
Personalized iPSC-derived dopamine progenitor cells have been used for PD therapy. The study reported that a 69-year-old man with a 10-year history of progressive PD underwent two separate surgeries for implantation of midbrain dopaminergic progenitor cells that had been differentiated in vitro from autologous iPSCs. Imaging suggested that the two grafts were delivered to the target sites and survived throughout the follow-up periods of 24 months (after the first surgery) and 18 months (after the second). No dyskinesias or other adverse neurological effects were reported.
Endothelial progenitor cells (EPCs) differentiate into the cells that make up the lining of blood vessels, and play roles in vasculogenesis and angiogenesis. iPSC-derived EPCs could be generated by using a protocol that involved the plating of iPSC embryoid bodies onto matrix-coated plates in the presence of BMP4, activin A, FGF2, VEGF and SB431542. Besides, it is feasible to generate iPSC-derived EPCs carrying an anti-tumorigenic therapeutic agent (CD40L) which targets tumors and inhibits their growth.
Inflammatory bowel diseases (IBDs) such as Crohn's disease (CD) and ulcerative colitis, are chronic relapsing diseases characterized by persistent intestinal inflammation and mucosal injuries. The iPSC-derived mesenchymal stem cells (iPSC-MSCs)-mediated immunosuppression is sufficient to increase peripheral CD4+ FOXP3+ Tregs, which regulate reparative and anti-inflammatory activities to alloimmune inflammation during rejection. Moreover, iPSC-MSCs not only act as ideal tools for pathological research but also provide platforms for drug screening and toxicity testing. Scientists have reported different iPSC-MSCs based studies for osteogenesis imperfecta (OI), Fanconi anemia (FA), Hutchinson-Gilford progeria syndrome, and fibrodysplasia ossificans progressiva (FOP) research.
Duchenne muscular dystrophy (DMD) is caused by mutations of the gene that encodes the protein dystrophin. Loss of dystrophin leads to severe and progressive muscle-wasting in both skeletal and heart muscles. Studies revealed that human iPSC-derived skeletal muscle progenitor cells promote myoangiogenesis and restore dystrophin in Duchenne muscular dystrophic mice.
Human iPSC-derived smooth muscle cells (SMC) promote angiogenesis and accelerate diabetic wound healing, making them a promising new candidate for the treatment of diabetic wounds. iPSC-SMC treatment is associated with an increased overall number of macrophages, with less M1 type and more M2 type macrophages, compared with acellular and murine ADSC wounds.
The differentiation of human iPSCs to the B-cell lymphoid lineage has important clinical applications that include in vitro modeling of developmental lymphogenesis in health and disease. iPSCs can be differentiated to functional tumor-targeting NK and T cells in vitro with demonstrable anti-tumor activity. Genetic modifications employed at the iPSC level can deliver desirable immunotherapeutic attributes to the generated immune effectors. iPSC-NK cells are currently evaluated in a clinical setting and pre-clinical testing of iPSC-T cells shows promising results but their production seems more challenging.
References
For Research Use Only. Not For Clinical Use.