All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
The prognostic performance of immunoscore to predict tumor staging has shown to be better than that of conventional TNM staging system and existing tumor risk parameters. Current immunohistochemical technologies allow the use of immunoscore assay in routine diagnostic pathology. Thus, considering the probable universal character of the immune control of tumors, it is essential to introduce the immunoscore as a component of cancer classification, taking into account the immune parameter as a prognostic factor.
With our state-of-the-art technology and industry-leading expertise, Creative Biolabs is versed in immunoscore assay for colorectal cancer (CRC). Our seasoned scientists continue to serve our clients from around the world with professionalism and expertise. We offer accurate and effective solutions for researchers who are committed to therapeutic development for CRC.
Cancer is a complex and dynamic disease characterized by major hallmarks. TNM classification is the most common system for classifying the extent of the spread of cancer. The tumor staging gives an estimation of the degree of tumor progression. The TNM classification is valuable in estimating the outcome of patients for a range of cancers and has been used for over 80 years. Although TNM staging system has stood the test of time, it provides incomplete prognostic information. The clinical outcome can significantly vary among patients within the same tumor stage.
A potential clinical translation of these observations is the establishment of a scoring system termed 'immunoscore', derived from the immune contexture and based on the enumeration of CD3+ and CD8+ lymphocyte populations. As a clinically useful Prognostic Biomarker in CRC, these two populations are in the core of the tumor and the invasive margin of tumors. Immunoscore has demonstrated its prognostic and predictive values in CRC and is now integrated into the guideline recommendations for patients.
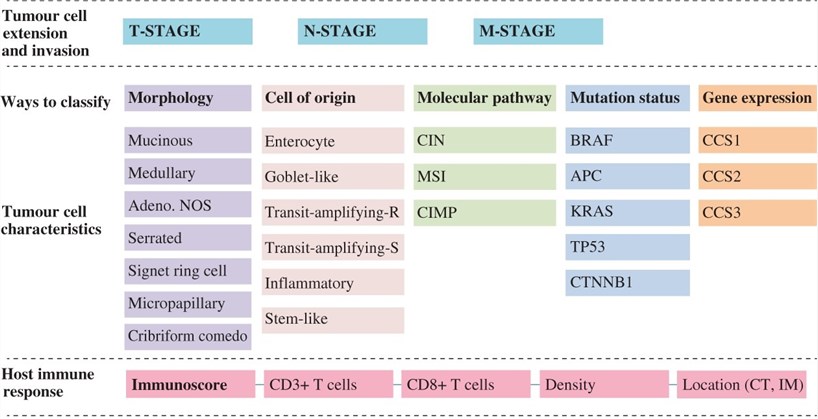 Fig.1 Classification of CRC. (Galon, 2014)
Fig.1 Classification of CRC. (Galon, 2014)
Adding immunoscore to a model combining all clinical variables significantly improve the prognostic accuracy. Creative Biolabs has a team of scientists, regulatory and quality staff that have highly experienced in Immune Biomarker Discovery. For prognostic biomarkers for CRC classification, immunoscore assay can help a lot. Immunoscore assay is a standardized immune assay based on quantification by digital pathology of CD3+ and CD8+ cytotoxic T cells in tumor tissues to accurately prognosticate CCR. Immunoscore assay offers a score ranging from immunoscore 0 when low densities of both CD3+ and CD8+ cell types are found in both regions, to immunoscore 4 when high densities are found in both regions. This assay has shown to be very powerful in CRC patients with clinically localized colorectal cancer, with no detectable tumor spreading to lymph nodes or distant organs.
By leveraging the wealth of information that we have in immunoscore assay, Creative Biolabs is pleased to help our clients with their study in CRC. For more detailed information, please feel free to contact us or directly send us an inquiry.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION