All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
CAR-T cells targeted to CD19 antigen on B cells have achieved great clinical results in B-cell acute lymphoblastic leukemia (B-ALL) treatment. Dual-target CAR-T cells were developed to decrease the possibility of relapse induced by antigen escape after receiving CD19 CAR-T therapy. Data from clinical trials showed that tandem CD19/CD22 CAR-T cells reached higher complete remission (CR) rates than single CD19 CAR-T cells and similar side effects. These results demonstrated that tandem CD19/CD22 CAR T-cell therapy is superior for B-ALL patients.
Although anti-CD19 chimeric antigen receptor (CAR) T-cell therapies have produced 68%–93% remission in r/r B-cell acute lymphoblastic leukemia (B-ALL) patients after adoptive transfer, the limited 6.1 months of event-free survival time and CD19-negative relapse (25%–42%) is still a challenge for CAR-T therapy application. CD22 is another B cell antigen that shows similar expression features to CD19 and is applied to CAR therapy. Scientists have developed various CAR-T therapies targeting CD19 and CD22, including single CD19 CAR-T, single CD22 CAR-T, tandem CD19-CD22 CAR-T, and sequential infusion of CD19 CAR-T and CD22 CAR-T cells (sequential CD19/CD22).
By comparing data from 219 patients who received CAR-T treatments from clinical trials between 2018 to 2021, researchers found that bispecific CD19-CD22 tandem CAR-T obtained better therapeutic function than single CD19 CAR-T, while similar to sequential CD19/CD22 group.
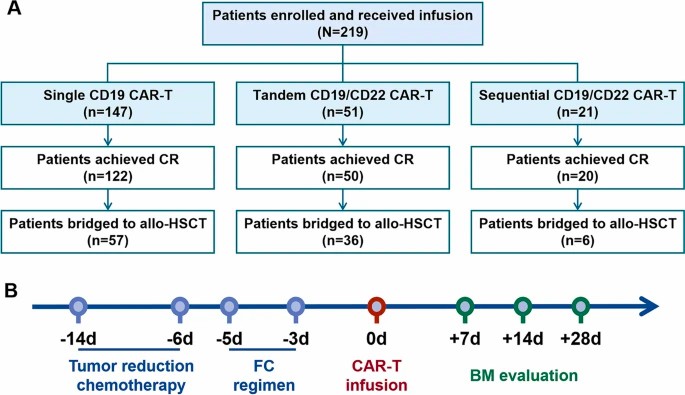 Fig.1 Timeline of CAR-T therapy and patients' information for retrospective study.1
Fig.1 Timeline of CAR-T therapy and patients' information for retrospective study.1
Scientists analyzed each strategy on CR rate and MRD-negative CR rate and found that tandem CD19-CD22 CAR-T treatment produced better clinical response than single CD19 (98% vs. 83%) and sequential CD19/CD22 CAR-T strategies (95.2%). The CR rate was especially significant for patients with High-risk cellular and genetic mutations (100% vs. 82.4%).
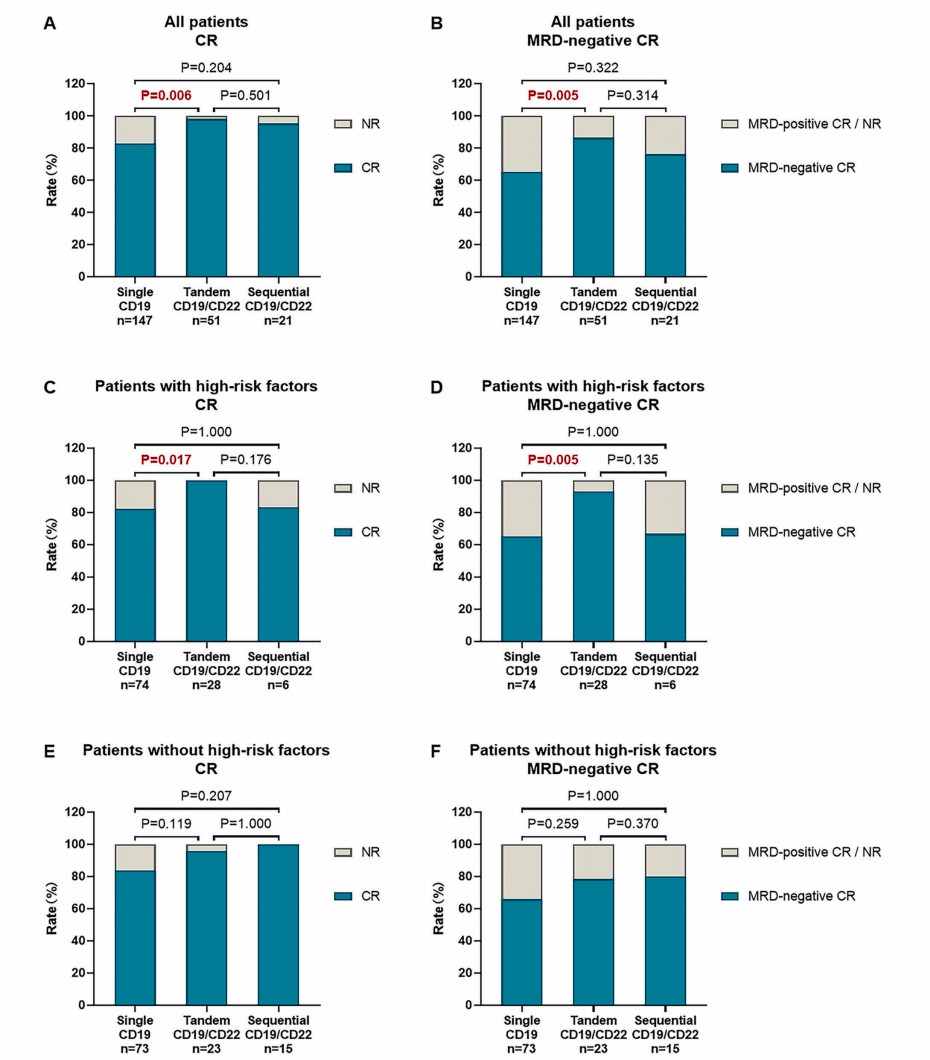 Fig.2 Data on response rate to three kinds of CAR-T strategies.1
Fig.2 Data on response rate to three kinds of CAR-T strategies.1
Moreover, the tandem CD19-CD22 CAR-T group shows a prolonged OS (overall survival) and median LFS (leukemia-free survival) time than the single CD19 CAR-T and sequential CD19 and cd20 group. As a potential bridging technique for leukemia treatment, dual-target CD19-CD22 produced significantly higher OS than a single CD19 CAR-T, and the LFS and CIR were the same between the three groups.
As for adverse events, different grades of CRS, ICANS, and cytotoxicity to other organs have occurred, but there was no significant difference in the three groups.
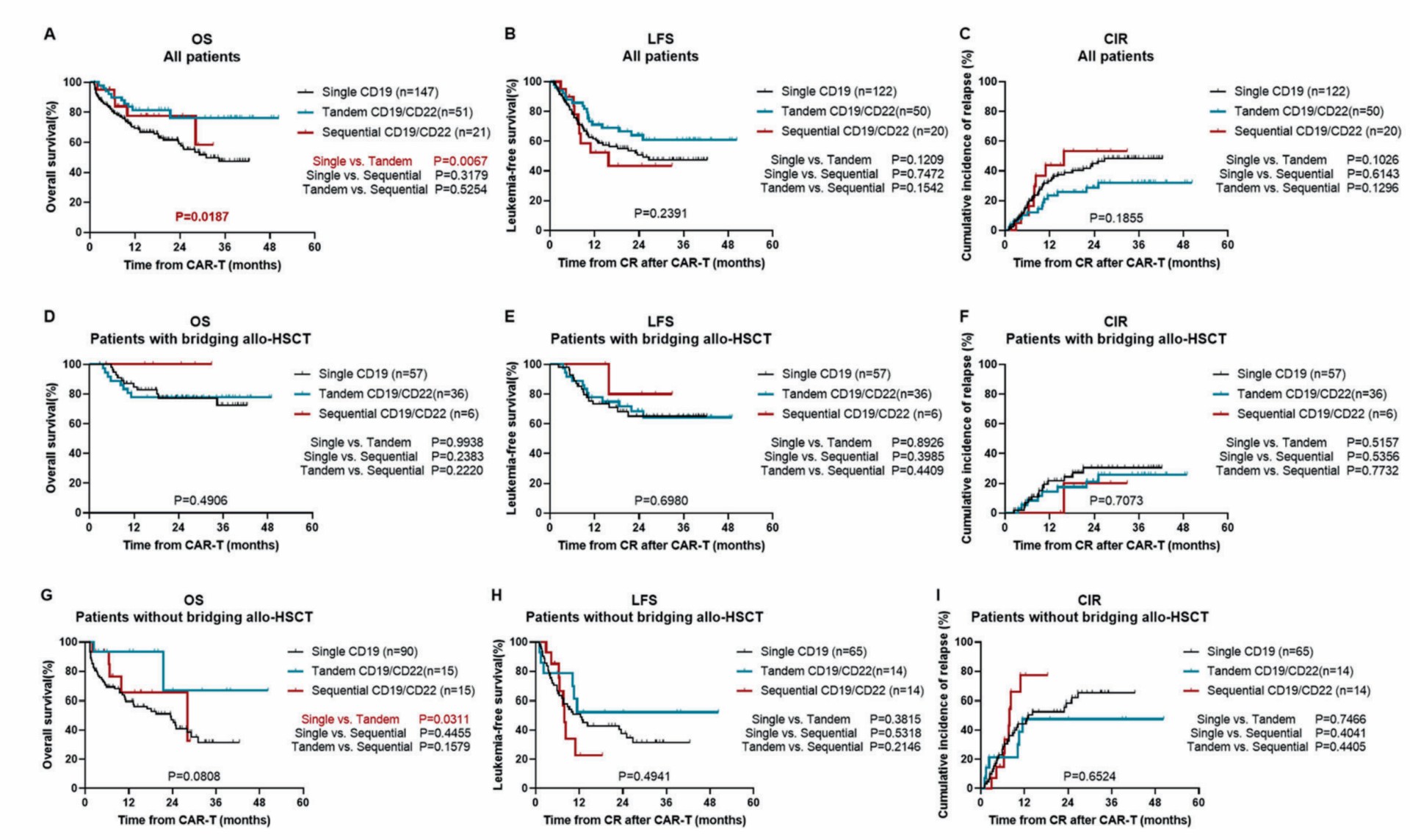 Fig.3 The survival rate of patients receiving different CAR-T cells.1
Fig.3 The survival rate of patients receiving different CAR-T cells.1
CAR-T immunotherapy has reached exciting progress in recent years in cancer treatment. With extensive successful cases in CAR-T construction and manufacturing, Creative Biolabs offers all types of products and one-stop services for CAR-T therapy, including innovative approaches with validated efficacy for development, efficacy test services, and clinical trials.
Biomarker Identification & Selection
A cancer biomarker is a critically differently expressed molecular on tumor cells. Creative Biolabs provides a large number of cancer targets and helps identify novel cancer-associated antigens for various cancer types.
CAR is the engineered receptor of immune cells that can specifically recognize cancer antigens. Creative Biolabs has a robust CAR design and construction platform that provides classical and special CAR construction services.
CAR-T Gene Packaging & Delivery
Creative Biolabs offers multiple methods and techniques to deliver CAR constructs into T cells, including virus-mediated transfection and various nucleofection methods with high efficiency and precision genome targeting.
Creative Biolabs provides various products and kits covering the whole process of immunotherapy development for multiple cancers and antigen targets, such as CAR vector products, CAR cell products, CAR viral particles, CAR animal cells, and so on. Our products are validated by our in-house scientists with high quality and are recognized by global clients.
CD22-Targeted Product Category
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION