All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Pharmacodynamic (PD) biomarkers are becoming increasingly valuable for assessing drug activity and target modulation in clinical trials. However, due to the increasing volume and heterogeneity of relevant data describing the biological networks that underlie disease mechanisms, identifying specific PD biomarkers is challenging and in the focus of many research groups. Integrating state-of-the-art technologies as well as industry-leading PD expertise, Creative Biolabs offers a comprehensive panel of assays for PD biomarker evaluation. Our scientific team has the necessary expertise and capabilities to provide overall solutions for PD biomarker discovery, focusing on innovative research.
The usefulness of biomarkers can be seen in many contexts such as guiding treatment decisions in the form of safety and efficacy biomarkers, as well as in the use of PD biomarkers. As clinical trials are designed for increasingly smaller, molecularly defined patient populations, there is a growing need for PD biomarkers in drug development. In particular, PD studies may provide insights into proof of mechanism, proof of concept, dose selection, and demonstrate drug mechanism of action. The development of PD biomarkers in oncology has implications for the design of clinical protocols from preclinical data and for predicting clinical outcomes from early clinical data.
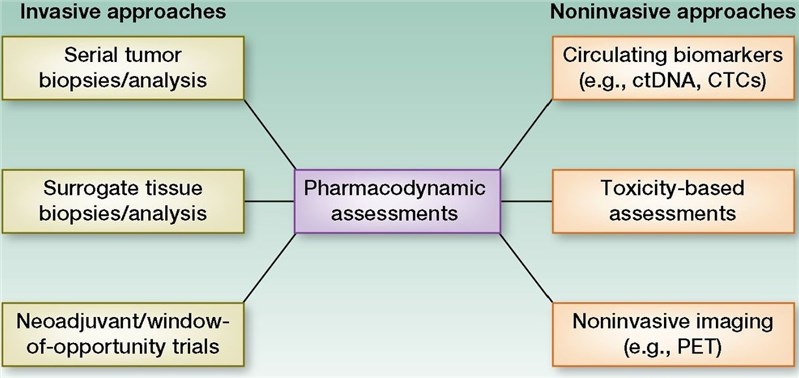
Fig.1 Depiction of various pharmacodynamic biomarker assessments. (Gainor, 2014)
The development of PD biomarkers is critically important in cancer drug development to examine whether drugs are modulating the intended therapeutic targets or pathways. Based on our advanced Immune Monitoring Platform, Creative Biolabs provides reliable PD assays that can help provide invaluable guidance within their drug development programs. Our scientists have developed multiple technology platforms across multiple sample types to assess PD biomarker not only in large molecule biotherapeutics but also in small molecule compounds. We have a broad range of analytical techniques for the detection of PD markers, including:
Including quantitative real-time RT-PCR and next-generation sequencing (NGS).
Including multiplex cytokine assays, Flow Cytometry (FC), Enzyme-linked Immunosorbent Assay (ELISA), Enzyme-linked Immunosorbent Spot (ELISpot), ECL, CLIA, RIA, Multiplex Immunohistochemistry (IHC), ISH, FISH, and others.
For detecting endogenous biomarkers at low levels in biological fluids, LC-MS/MS provides superior selectivity and accuracy. Our seasoned scientists have developed PD biomarker assays to perform safety and efficacy testing of multiple compound classes, such as estradiol, total testosterone, cortisol, cortisone, citrulline, vitamin, resveratrol, cholesterol, fatty acid, and neurotransmitter.
To assist our clients' specific objectives of PD biomarker discovery, Creative Biolabs is proud to offer the most comprehensive services. We offer turn-key or ala carte services customized to our client's needs. Please feel free to contact us or directly send us an inquiry.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION