Pluripotent stem cells hold promise in the field of regenerative medicine. Because they can propagate indefinitely, as well as give rise to every other cell type in the body (such as neurons, heart, pancreatic, and liver cells), they represent a single source of cells that could be used to replace those lost to damage or disease. Induced pluripotent stem cells (iPSCs/iPS) are a type of pluripotent stem cells that can be generated directly from a somatic cell. iPSC is derived from skin or blood cells that have been reprogrammed back into an embryonic-like pluripotent state that enables the development of an unlimited source of any type of human cells needed for therapeutic purposes. These unlimited supplies of autologous cells could be used to generate transplants without the risk of immune rejection.
Leukemia forms in blood-forming tissue. The disease is characterized by the uncontrolled growth of blood cells, usually white blood cells in the bone marrow. White blood cells are a fundamental component of the body's immune response. Leukemia cells crowd out and replace normal blood and marrow cells.
Theoretical and technical inability in early screening and distinguishing cancer, tumor tolerance to common treatment methods, repeated relapses of cancer after remission phase, heterogeneous chromosomal abnormality, and the side effects of current chemotherapies are some of the challenges that we face with leukemia and other malignancies. iPSCs opened a promising window to a wide range of diseases, including leukemia. Many researchers have been trying to produce induced hematopoietic stem cells (iHSC) derived from iPSC.
Anemia is a condition in which you lack enough healthy red blood cells to carry adequate oxygen to the body's tissues. Anemia can be temporary or long term, and it can range from mild to severe. Anemia is a condition in which hemoglobin (Hb) concentration and/or red blood cell (RBC) numbers are lower than normal and insufficient to meet an individual's physiological needs. There are many causes of anemia, such as iron deficiency, bleeding, hemolysis, hematopoietic dysfunction, parasitic infection, and so on.
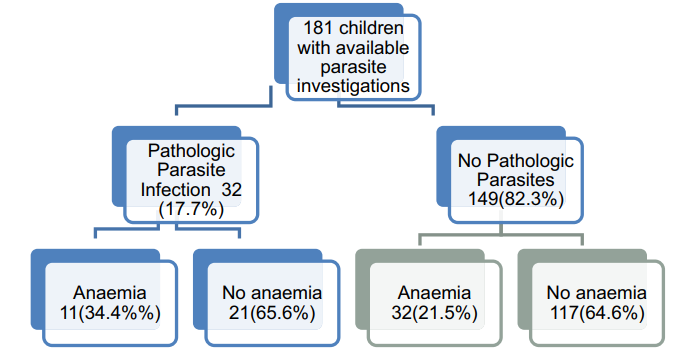 Fig.1 Profile of children with anemia and parasite infection.1
Fig.1 Profile of children with anemia and parasite infection.1
The innovation of reprogramming somatic cells to iPSC provides a possible new approach to treat thalassemia and other genetic diseases such as sickle cell anemia. iPSCs can be made from patients' somatic cells. The mutation in the globin gene can be corrected by gene targeting, and then differentiated into hematopoietic cells to be returned to the patient. Scientists reprogrammed the skin fibroblasts of a patient with homozygous thalassemia into iPSCs and showed that the iPSCs could be differentiated into hematopoietic cells that synthesized hemoglobin.
Erythropoietin (EPO), a potent regulator of erythropoiesis, is secreted by the kidney and liver. The kidney is the major site of EPO production in adults, and the liver is the primary organ of EPO production in the fetal and early neonatal periods, but EPO is produced by the adult liver in states of severe anemia. EPO production is reduced in patients with chronic kidney disease (CKD), eventually resulting in renal anemia. The differentiation of iPSC and embryonic stem cells (ESCs), which have unlimited self-renewal capability and the potential to differentiate into any cell type in the body, provides promising cell sources for regenerative medicine. The transplantation of hiPSC-EPO cells reversed renal anemia, with a return to the normal range for the hematocrit in immunodeficient mice. Therefore, EPO cells generated from hiPSCs/ESCs may be useful as a cell therapy for treating renal anemia.
AA is an acquired bone marrow failure syndrome characterized by pancytopenia and bone marrow hypoplasia. Patients often become dependent on blood and platelet transfusions and are at risk for significant infections from neutropenia and leukopenia. The natural history of untreated AA is death, most commonly from infection or hemorrhage.
Recent progress in human iPSCs has opened the door to better understanding the biology of human diseases, especially rare disorders such as acquired aplastic anemia, in which the target hematopoietic tissues are depleted. The advent of somatic cell reprogramming has presented new routes for generating hematopoietic stem cells from patient-derived iPSCs and their differentiation into hematopoietic lineages.
HA is the most common severe inherited bleeding disorder. This pathology exhibits different phenotypic expressions depending on its associated factor VIII (FVIII) plasma levels, resulting in severe (<1%), moderate (1-5%), or mild (6-30%) forms of expression. This disease originates from an inherited deficiency or dysfunction in the procoagulant FVIII, a crucial element of the intrinsic pathway of blood coagulation involved in the conversion of factor X to Xa. In severe cases, FVIII deficiency leads to spontaneous bleeding and internal hemorrhage that can cause disability and even death, if left untreated.
iPSCs could differentiate into different cells including hematopoietic stem and progenitor cells (HSPC). However, in vitro generation of transplantable HSPC from iPSC is still challenging. Recent studies reported that engraftable HSPC could be isolated from teratomas formed from mouse and human iPSC. Therefore, it would be worth exploring whether in vivo generated HSPC are functional for HSPC-based gene therapy. Anti-factor VIII (FVIII) neutralizing antibody (inhibitors) development is a serious problem in the replacement therapy of hemophilia A (HA). Platelet-targeted gene therapy is a promising approach for HA with inhibitors. FVIII-releasing platelets generated from FVIII modified HSPC could ameliorate the hemorrhage diathesis in the presence of inhibitors. Cas protein-based gene editing strategy provides a convenient method for targeted integration of a therapeutic gene to cure genetic diseases. Meanwhile, the genome editing of iPSC is easier and more efficient than that of HSPC, because iPSC can be easily proliferated and screened.
There are still many unsolved challenges in the treatment and clinical application of different types of stem cells. However, with the in-depth study of stem cells, it is not difficult to find that stem cells have great advantages and development space in the treatment of diseases. With years of experience in stem cell therapy development, Creative Biolabs provides high-quality stem cell research services to clients around the world.
Reference
For Research Use Only. Not For Clinical Use.