Induced pluripotent stem cells (iPSCs) exhibit stable self-renewal in culture and have the potential to differentiate into all somatic cell types. Differentiated cell types produced from a patient's iPSCs have many potential therapeutic applications, including tissue replacement and gene therapy, and as an in vitro model system to study human development and diseases. Creative Biolabs has broad expertise in lineage-specific iPSC differentiation and here we introduce our custom digestive differentiation services.
iPSCs are a type of stem cell that can be generated directly from adult cells. These cells have the potential to differentiate into a variety of different cell types, including cells of the digestive system such as the intestines, liver, and pancreas.
To date, iPSCs have been successfully differentiated into several types of digestive system cells, including:
While the potential applications of iPSC-derived digestive system cells are broad, a great deal of research is still needed to ensure the safety and efficacy. Additionally, developing reliable methods for generating large numbers of high-quality, functional cells reproducibly is a major focus of ongoing research.
iPSCs have the ability to differentiate into pancreatic β cells under specific induced culture conditions. It has proved to be a promising approach for stem cell-based diabetes therapy. Efforts are made to better control the differentiation process and increase efficiency under in vitro culture conditions, including the use of various combinations of transcription factors, small molecules, and growth factors. Stepwise differentiation protocols have been developed to generate mature β cells from iPSCs, which include 4 steps: definitive endoderm (DE), pancreatic progenitors, pancreatic endocrine progenitors, and β cells. For each step, the cells are treated with different reagents.

The differentiation of iPSCs into specific cell types of the intestinal cells would provide insights to the understanding of intestinal development and ultimately yield cells for the use in future regenerative medicine. To promote the studies in this field, Creative Biolabs now offers directed in vitro differentiation of iPSCS into four intestinal differentiated cell types, namely absorptive enterocytes, goblet cells, enteroendocrine cells, and Paneth cells.
At Creative Biolabs, we provide not only in vitro differentiation services but also standardized in vitro and in vivo testing to assess the morphology and functionality of the differentiated cells.
In addition, you might be also interested in other iPSC differentiation services listed below:
Custom differentiation into other cell types not listed above is available upon request. Creative Biolabs has accumulated multiple successful experiences with diverse custom projects in the past years. Please contact us for further information.
Below are the findings presented in the article related to digestive system-cells differentiation from iPSC.
1. Researchers developed an effective differentiation method to induce human iPSC to form endoderm. Using the ACP (Activin A, CHIR99021 and PI-103) protocol, hiPSC-derived endoderm was differentiated into hepatocytes. Differentiated hepatocytes showed higher gene expression levels of albumin (ALB), hepatocyte markers, bile salt efflux pump (BSEP), and CYP3A4 than those differentiated using conventional methods. And the hepatocytes showed CYP3A4/5 activity, and the metabolic activity of ACP hepatocytes was significantly higher than that of conventionally differentiated hepatocytes. These studies suggest that human iPSC-derived endoderm can be further differentiated into hepatocytes with pharmacokinetic functions.
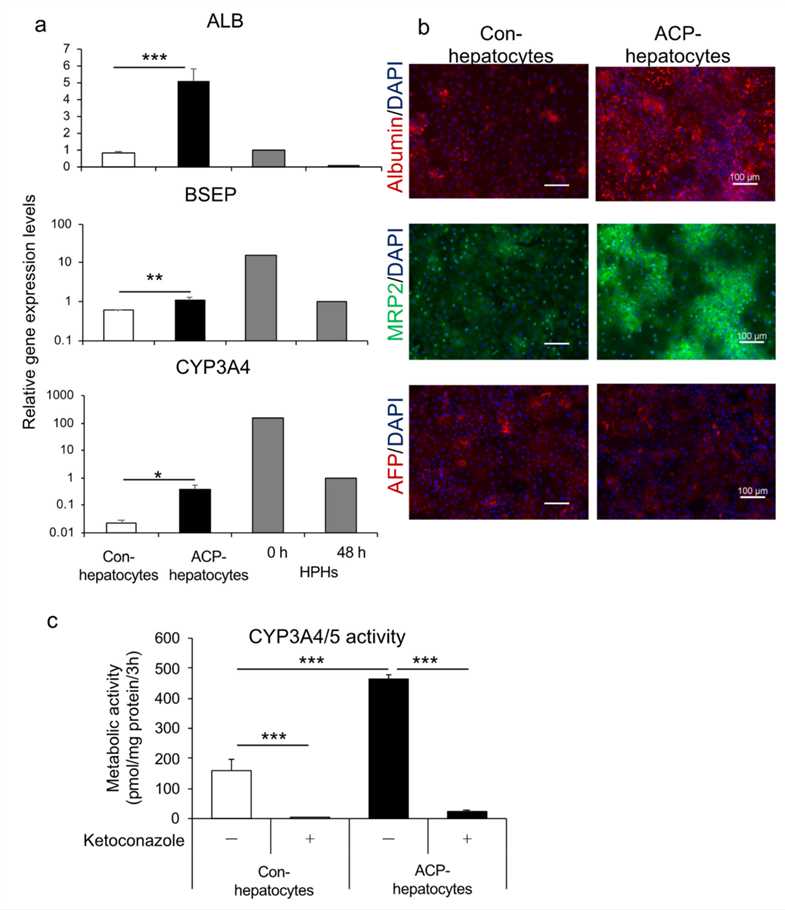 Fig. 1 Characterization of hepatocytes using hiPSC-derived endoderm generated by protocol ACP.1
Fig. 1 Characterization of hepatocytes using hiPSC-derived endoderm generated by protocol ACP.1
2. Researchers differentiated human iPSC into pancreatic β-cells and characterized the transcriptional dynamics throughout the differentiation process. Gene expression analysis of the bulk cells showed that pluripotency genes OCT4 and NANOG were downregulated, while pancreatic development genes such as SOX17, FOXA2, HNF1b, PDX1, NKX2.2, and NKX6.1 were significantly upregulated during differentiation.
Immunofluorescence also confirmed the expression of β-cell markers in iPSC-derived insulin-positive cells. Secretion of C peptide, glucagon, and growth hormone-releasing peptide was measured in supernatants and assessed in unstimulated cells at the differentiation stage, which rapidly responded to glucose stimulation. Taken together, these data suggest that iPSCs have the ability to differentiate into bona fide β-cells that are capable of secreting insulin in response to glucose.
Reference
For Research Use Only. Not For Clinical Use.