Creative Biolabs has established an innovative and excellent platform for the discovery of anti-parasite biomolecular. The Anti-Parasitic Biomolecular Discovery can offer high affinity antibodies and peptides targeting Plasmodium which are suitable for scientific researches and clinical applications.
Infection with parasites of the genus Plasmodium leads to Malaria, which remains a major public health problem in large parts of sub-Saharan Africa, tropical and subtropical regions of Asia and the Americas. P. falciparum is the most lethal species that is responsible for almost all the Plasmodium-induced mortality. Sporozoite-stage malarial parasites are inoculated by an infected female Anopheles mosquito rapidly invade hepatocytes. After five to six days, the parasites become mature liver-stage schizonts with thousands of merozoites. When the hepatocyte ruptures, the merozoites are released into the blood stream. Each of these merozoites can invade an erythrocyte, initiating the erythrocytic stage of the parasite's life cycle.
Apical membrane antigen 1 (AMA1) released from the parasite micronemes is a type I integral membrane protein. It is synthesized in segmenting schizonts as an 83 kDa precursor protein. It has been revealed that the interaction between AMA1 and another parasite protein called rhoptry neck protein 2 (RON2) is required for the merozoite invasion. Blocking the AMA1-RON2 interaction is a promising strategy for anti-Plasmodium therapy. For example, both peptide RON2L and AMA1-binding monoclonal antibodies can compete with native RON2 protein to interact with AMA1, leading to inhibition of merozoite invasion.
Erythrocyte binding antigen-175 (EBA-175) is a P. falciparum parasite ligand that binds to its receptor glycophorin A (GpA) on human erythrocytes which is heavily sialylated. Within EBA-175, region II (RII) is sufficient for GpA binding and is comprised of two Duffy Binding Like (DBL) domains, F1 and F2. This binding event is necessary for P. falciparum invasion to erythrocyte. EBA-175 has been revealed as a leading vaccine candidate. Blocking EBA-175/GpA is a potential way for the development of anti-P. falciparumagent therapies.
P.falciparum reticulocyte binding protein homologue 5 (PfRH5) belongs to the superfamily of erythrocyte ligands referred to as the reticulocyte binding like proteins (RBLs). Basigin (BSG) has been identified as the receptor for PfRH5. PfRH5 interacting with BSG is essential and universally required for P. falciparum invasion to erythrocyte. PfRH5 has been identified as an effective target for the development of antibody and vaccine against P. falciparum. Anti-PfRH5 monoclonal antibodies were shown to inhibit PfRH5-BSG interaction, exerting a broad neutralizing effect in vivo study.
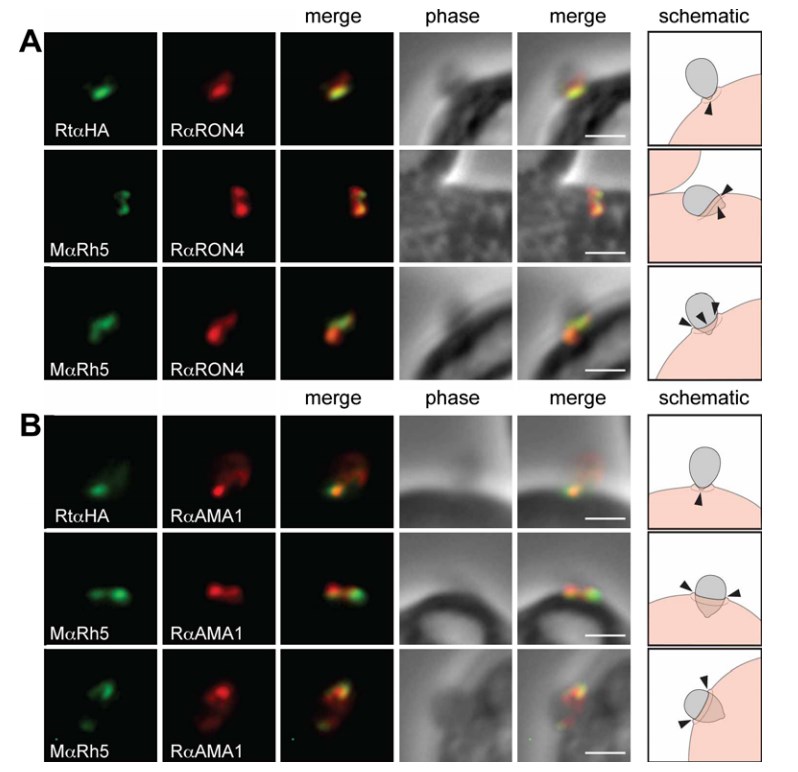 Fig.1 PfRh5 follows the tight junction during invasion. Co-localisation of PfRh5 with rabbit antisera against two markers of the tight junction (A) RON4 and (B) AMA1 in invading merozoites arrested using cytochalasin D. Cartoon schematic is shown on the right, with black arrows marking tight junction. DAPI nuclear stain. Scale bars = 1 lM. (Baum et al. 2009)
Fig.1 PfRh5 follows the tight junction during invasion. Co-localisation of PfRh5 with rabbit antisera against two markers of the tight junction (A) RON4 and (B) AMA1 in invading merozoites arrested using cytochalasin D. Cartoon schematic is shown on the right, with black arrows marking tight junction. DAPI nuclear stain. Scale bars = 1 lM. (Baum et al. 2009)
With years of experience in the field of antibody development, Creative Biolabs has successfully developed an advanced AntInfectTM Platform, which is optimal for neutralizing antibody generation. The anti-Plasmodium biomolecular generation can be achieved depending on known targets as listed above as well as novel targets. We have comprehensive biomolecular development such as phage display, hybridoma technology. Our seasoned scientists can one-stop customized biomolecular development service for all our global customers. Please contact us for more information and a detailed quote.
Reference