Sequence Liability Identification Service
Overview Publication Why Choose Us FAQs Customer Review Related Services Contact Us
Overview
As a leading solution provider who is focused on therapeutic antibody discovery and development, Creative Biolabs has established a powerful antibody engineering platform including reformatting and production, epitope binning and mapping, antibody humanization platform, antidboy maturation, measurement, sequence liability identification, immunogenicity prediction, therapeutic developability analysis and other antibody specialization and modification services.
Sequence Liability Identification Service
Post-translational modifications (PTMs) of antibody have the potential to affect affinity, stability, potency and homogeneity of an antibody, and this will result in complicated process in downstream development. The bioactivity and production of various isoforms of the product will be impacted. PTMs normally include deamidation, isomerization, oxidation, glycosylation, free thiol, pyro-Glutamate, C-terminal Lysine etc.
To identify the sequences within antibody variable regions, Creative Biolabs has developed algorithms to offer this kind of service to improve the potential product quality. We are dedicated to helping our clients to make smarter decisions about eliminating the program earlier in the antibody engineering process or selecting the liability free antibodies for further development. We aim to provide critical insight into your project and reduce your cost via foreseeing potential quality risks.
|
Scope
|
We provide sequence liability identification service for variable heavy and variable light chains.
The following PTMFs can be analyzed including, but not limited to, N-linked glycosylation, deamidation, isomerization, oxidation, pyro-glutamate formation, and c-terminal lysine.
|
|
Material needed
|
Before performing sequence liability identification service, please deliver amino acid sequence of finalized heavy and light chain variable regions.
|
|
Deliverables
|
Final report with potential sequence liabilities will be analyzed and sent to you.
|
We also provide relevant antibody sequencing services:
-
Antibody variable region sequencing and cloning;
-
N-terminal protein sequencing service;
-
De novo antibody sequencing.
Publication
This publication introduces a new bioinformatics tool called the liability antibody profiler (LAP). This tool aims to de-risk therapeutic antibody candidates by identifying and prioritizing potential chemical liability motifs in their amino acid sequences and structures. The paper notes that a simple sequence-based annotation of these liabilities is often over-predictive, as most antibodies contain an average of 3-4 such motifs regardless of their source.
To address this issue, LAP incorporates three computational flags to help prioritize liabilities that are more likely to cause problems:
-
Germline: To identify naturally occurring motifs.
-
Therapeutic: To reflect motifs found in successful therapeutic antibodies.
-
Surface: To indicate structural accessibility for chemical modification.
The study shows that these flags can annotate approximately 60% of liability motifs as benign, meaning they have a lower probability of degradation. The LAP tool is publicly available online and is intended to save time and effort in the de-risking process of therapeutic molecules. The publication also includes a detailed reference for various antibody liabilities, their severity, and descriptions.
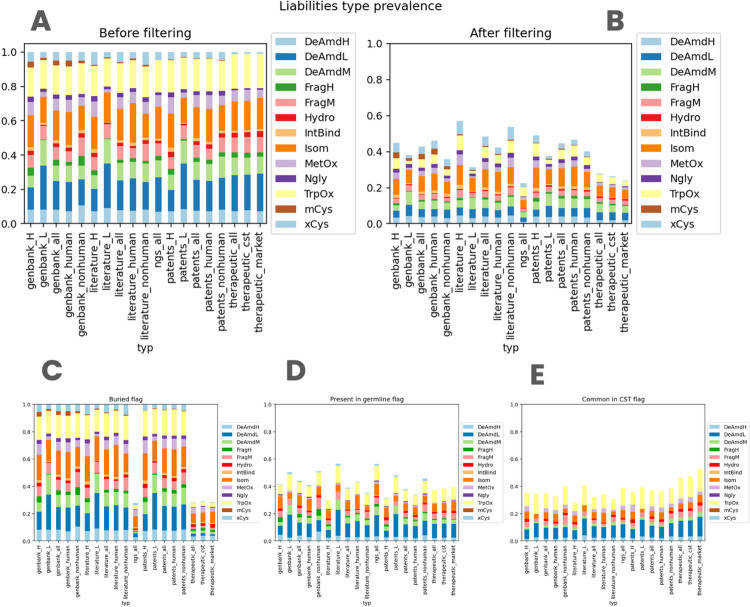 Fig.1 Assessment of sequence-based liability prevalence across diverse datasets.1
Fig.1 Assessment of sequence-based liability prevalence across diverse datasets.1
Why Choose Creative Biolabs?
Creative Biolabs stands at the forefront of biopharmaceutical innovation, offering unparalleled expertise in sequence liability identification. Our commitment to scientific excellence, combined with our integrated platform, provides distinct advantages that set us apart. We don't just identify problems; we offer robust, data-driven solutions that accelerate your development, enhance product quality, and facilitate regulatory success. Our years of experience and deep scientific knowledge ensure your project is in expert hands.
Experience the Creative Biolabs Advantage - Get a Quote Today.
Your Questions Answered
Q1: What types of sequence liabilities can Creative Biolabs identify?
A1: Creative Biolabs covers a comprehensive range of sequence liabilities, including deamidation, oxidation, and aggregation-prone regions. Our advanced platform provides a thorough analysis of your molecule's vulnerabilities, ensuring a complete risk profile for a wide range of potential issues.
Q2: How accurate are your in silico predictions for sequence liabilities?
A2: Our in silico predictions leverage Creative Biolabs' proprietary algorithms and extensive databases. We use intelligent filtering flags (germline presence, therapeutic occurrence, and structural accessibility) to reduce false positives, allowing us to focus on the most relevant liabilities for efficient experimental validation.
Q3: What if my protein has a unique or unconventional sequence?
A3: Creative Biolabs' platform handles diverse protein sequences, including novel structures. Our experts specialize in custom analysis, combining standard protocols with tailored approaches to address the specific challenges of your unique biotherapeutic molecule. We are equipped for even the most complex biotherapeutics.
Customer Reviews: Voices of Our Scientific Partners
-
Reduced Aggregation Issues
Using Creative Biolabs for sequence liability identification significantly improved our understanding of protein aggregation. Their detailed reports pinpointed specific residues, enabling successful mutations that reduced aggregation during purification and storage. This service proved invaluable for early-stage development and directly boosted our project's efficiency. - Dr. La S*h
-
Enhanced Immunogenicity Prediction
Creative Biolabs provided a thorough analysis of potential immunogenic epitopes in our fusion protein. Their comprehensive approach, combining in silico prediction with rigorous in vitro validation, gave us immense confidence in the candidate's safety profile, guiding our development decisions. - Dr. Pl J*s
Related Services: Comprehensive Support for Biotherapeutic Development
We also provide relevant antibody sequencing services:
Variable Region Sequencing and Cloning
Antibody variable region (VH and VL) sequencing and cloning are crucial for antibody construction. Creative Biolabs provides a one-stop solution for therapeutic antibody discovery by offering this essential first step: variable region sequencing and cloning services.
Learn More →
Sequence Liability Identification
Creative Biolabs, a therapeutic antibody discovery leader, offers a powerful engineering platform. Services include reformatting, production, epitope binning/mapping, humanization, maturation, affinity measurement, sequence liability identification, immunogenicity prediction, and developability analysis.
Learn More →
Ultrafiltration Assay
Ultrafiltration is a pressure-driven purification process that retains macromolecules while allowing water and small substances to permeate a membrane. Creative Biolabs provides ultrafiltration assays to remove endotoxins from ultrapure water using proprietary cartridges.
Learn More →
Contact Us
With extensive experience in providing antibody engineering solutions, Creative Biolabs is committed to excellence in sequence liability identification service and others. We aim to help you get high-quality antibodies for therapeutic product development and make smart decisions on whether or not to go. For more detailed information, please feel free to contact us at your convenience.
Reference
-
Satława, Tadeusz, et al. "LAP: Liability Antibody Profiler by sequence & structural mapping of natural and therapeutic antibodies." PLOS Computational Biology 20.3 (2024): e1011881. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.1371/journal.pcbi.1011881


 Fig.1 Assessment of sequence-based liability prevalence across diverse datasets.1
Fig.1 Assessment of sequence-based liability prevalence across diverse datasets.1
