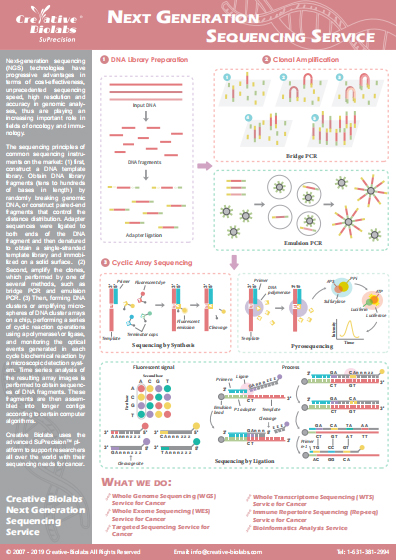
Tumor Mutational Burden (TMB) Analysis Service for Checkpoint Immunotherapy Response
Tumor mutation burden (TMB) is a prediction of the number of mutations carried by tumor cells and has been paid more and more attention in biomarker research. By measuring TMB, the cancer cell now has a unique fingerprint of natural and mutated proteins. Based on our cutting-edge SuPrecision™ platform, Creative Biolabs has accumulated extensive experience in performing whole exome sequencing (WES) and comprehensive analysis. Our scientists have developed a novel algorithmic approach for TMB analysis for response to checkpoint inhibitor immunotherapy. By comparing DNA sequences from a patient’s healthy tissues and tumor cells, our scientists can determine the number of acquired somatic mutations present in tumors but not in normal tissues. We are confident in providing clients with the best service at the most competitive cost.
Clinical Significance of TMB
-Analysis-for-Response-to-Checkpoint-Immunotherapy-1.jpg)
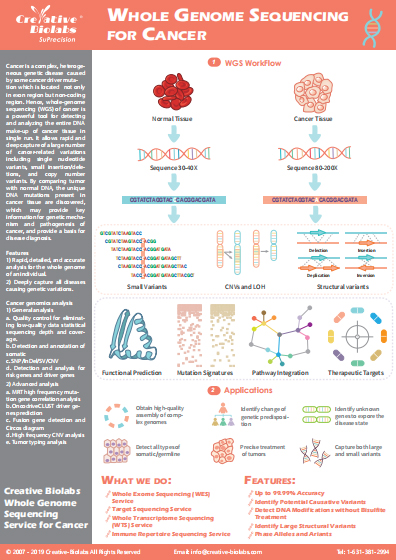
Cancer immunotherapy has revolutionized how we view cancer treatment. When it works, the results are transformative. It has been much more helpful in the improvement of the survival rate of many different cancers. Still, immunotherapies may not work for everyone. Only a subset of people treated with immunotherapies respond. Knowing who is likely to respond to immunotherapy will help ensure that these promising treatments deliver the most benefit. More informed treatment selection has the potential to steer patients away from ineffective medicines, which may reduce side effects and save both costs and disappointment. TMB is a genomic biomarker, which will potentially make a significant impact on the landscape of cancer immunotherapy, especially immune checkpoint response. As an emerging marker, TMB measures the number of mutations within a tumor genome and has already been shown to be associated with improved responses to checkpoint inhibitor immunotherapies in lung cancer, melanoma and bladder cancer. Most cancer biomarkers for immunotherapies are specific to certain immune proteins expressed by the tumor, but in contrast, TMB is derived solely from mutations. Importantly, TMB is consistently reproducible. It provides a quantitative measure that can be used to better inform treatment decisions, such as selection of targeted immunotherapies or enrollment in clinical trials.
TMB Analysis in Creative Biolabs
-Analysis-for-Response-to-Checkpoint-Immunotherapy-2.jpg)
Based on our advanced SuPrecision™ platform, WES is performed on tumors and normal tissue samples. Our scientists measure the number of non-synonymous DNA coding sequence changes per megabase of DNA obtained by WES. Our scientists have developed a novel algorithmic approach to measure the TMB value. The TMB value is determined across a wide variety of tumor types in an internal validation study. A detailed description of low, high intermediate, and high upper quartile in reference to the median genomic TMB value will be presented in the report.
Checkpoint blockade acts by activating the immune system to eliminate tumors. Checkpoint-blockade therapies have shown impressive increases in overall survival. Costimulatory molecules can promote or inhibit T cell receptor-mediated activation playing an important role in fine-tuning TCR-mediated T cell functions. Creative Biolabs can provide the most comprehensive TMB analysis for response to checkpoint immunotherapy. The checkpoint immunotherapy includes Co-inhibitory Immune Checkpoint Pathways and Co-stimulatory Immune Checkpoint Pathways.
|
Co-inhibitory Immune Checkpoint Pathways Including but not Limited to: |
Co-stimulatory Immune Checkpoint Pathways Including but not Limited to: |
|
|
Checkpoint Immunotherapy Has Shown Promise in Treating a Variety of Diseases, Including but not Limited to:
- Lung cancer
- Melanoma
- Bladder cancer
- Ovarian cancer
- Kidney cancer
- Hodgkin’s lymphoma
- Breast cancer
Key Advantages of Our TMB Analysis Service Including but Not Limited to:
- Fully automated library preparation
- Sequencing on state-of-the-art sequencers, with paired-end 100 bp
- A large variety of bioinformatic analysis options
- High-quality results and detailed project report
- Fast turnaround time
With years of research and development experience in the field of next-generation sequencing (NGS) technology, Creative Biolabs has accumulated extensive experiences in WES for TMB analysis. We can provide top-quality service of TMB analysis the most competitive cost.
Please contact us for more information and a detailed quote.
Reference
- Xu, Ning, et al. "Integrated proteogenomic characterization of urothelial carcinoma of the bladder." Journal of hematology & oncology 15.1 (2022): 76. Distributed under open access license CC BY 4.0, without modification.
Q&As
Resources
Infographics
Podcast
- WES based Variant Analysis
- WES based Structural Variant Detection
- WES based CNV Detection
- WES based Tumor-Specific Neoantigen Discovery
- WES based Biomarker Discovery
- Personal Tumor-Specific Neoantigen Vaccine Development
- MRD Monitoring
- Microsatellite Instability Analysis
- Immune Repertoire Germline Gene & Allele Identification
- One-Stop Cancer 3D Modeling
- Circle-Seq based eccDNA Identification
- RNA-Seq based Tumor Microenvironment Analysis